Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 54(5); 2020 > Article
-
Original Article
A retrospective cytohistological correlation of fine-needle aspiration cytology with classification by the Milan System for Reporting Salivary Gland Cytopathology -
Ji Hyun Park1
 , Yoon Jin Cha1
, Yoon Jin Cha1 , Ja Yeong Seo1
, Ja Yeong Seo1 , Jae Yol Lim2
, Jae Yol Lim2 , Soon Won Hong1
, Soon Won Hong1
-
Journal of Pathology and Translational Medicine 2020;54(5):419-425.
DOI: https://doi.org/10.4132/jptm.2020.06.09
Published online: July 8, 2020
1Department of Pathology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
2Department of Otorhinolaryngology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- Corresponding Author: Soon Won Hong, MD, PhD, Department of Pathology, Gangnam Severance Hospital, Yonsei University College of Medicine, 211 Eonju-ro, Gangnam-gu, Seoul 06273, Korea Tel: +82-2-2019-3540, Fax: +82-2-2019-3540, E-mail: soonwonh@yuhs.ac
© 2020 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Before publication of the new classification system named the Milan System for Reporting Salivary Gland Cytopathology (MSRSGC) in 2018, there was no standard classification for salivary gland lesions obtained by fine-needle aspiration (FNA). We therefore aimed to evaluate the diagnostic utility of this system by retrospectively reviewing FNA samples using the MSRSGC and to determine their risk of developing into neoplasms and becoming malignant.
-
Methods
- Retrospective slide review and classification of salivary gland FNAs obtained over a 6-year period (2013–2018) at a single center were performed by two pathologists. The risks of neoplasm and malignancy for each category also were calculated.
-
Results
- This study surveyed 374 FNAs (371 patients) performed over a six-year period and selected 148 cases that included documented surgical follow-up (39.6%). Among the surgically treated cases, the distributions of FNA categories were as follows: non-diagnostic (ND; 16.9%), non-neoplastic (NN; 2.7%), atypia of undetermined significance (AUS; 3.4%), benign (BN; 54.7%), salivary gland neoplasm of uncertain malignant potential (SUMP; 10.1%), suspicious for malignancy (SM; 6.8%), and malignant (M; 5.4%). The risk of malignancy (ROM) was 24.0% for ND, 0% for NN, 40.0% for AUS, 2.5% for BN, 46.7% for SUMP, 100% for SM, and 87.5% for M. The overall diagnostic accuracy was 95.9% (142/148 cases).
-
Conclusions
- The newly proposed MSRSGC appears to be a reliable system for classification of salivary gland lesions according to the associated ROM.
- Research objective
- A retrospective search of the cytopathology database from the past six years (January 2013-December 2018) for salivary gland (all major and minor salivary glands) FNA specimens at Gangnam Severance Hospital, School of Medicine, Yonsei University was performed. Clinical data regarding age, sex, and location of the lesion, as well as the type of tumor, were collected from patient medical records. Follow-up histopathological reports were also obtained if available. From January 2013 to December 2018, 374 FNAs were performed, and 150 of these also underwent surgical resection. Among them, 148 cases were finally enrolled in this study; two cases were excluded as they did not meet the inclusion criteria.
- The FNAs were performed via a direct percutaneous or transoral route using a 23-gauge needle. The smears were then fixed in 95% ethanol for Papanicolaou staining, which was performed in the cytopathology laboratory.
- Slide review and categorization
- Blinded review of all FNA slides was carried out by two pathologists (J.H.P. and Y.J.C.), and each case was assigned to an MSRSGC category. When there was a diagnostic discrepancy, the two pathologists had a discussion to decide upon the best MSRSGC category. Category I cases were further divided into an inadequate group and a cyst-contents-only group. Matched slides of surgical specimens were also examined, and the histological diagnoses of these surgical specimens were categorized as NN, BN, or M.
- Evaluation of risk of malignancy and risk of neoplasm
- Cytologic-histologic correlations were performed to determine risk of malignancy (ROM) and risk of neoplasm (RON). The ROM was defined as the ratio between the number of FNAs and the number of surgically confirmed malignancies. Similarly, the RON was defined as the ratio between the number of FNAs and the number of neoplasms, including both benign and malignant neoplasms. The ROM and RON values were calculated for each MSRSGC category.
MATERIALS AND METHODS
- Basal patient characteristics
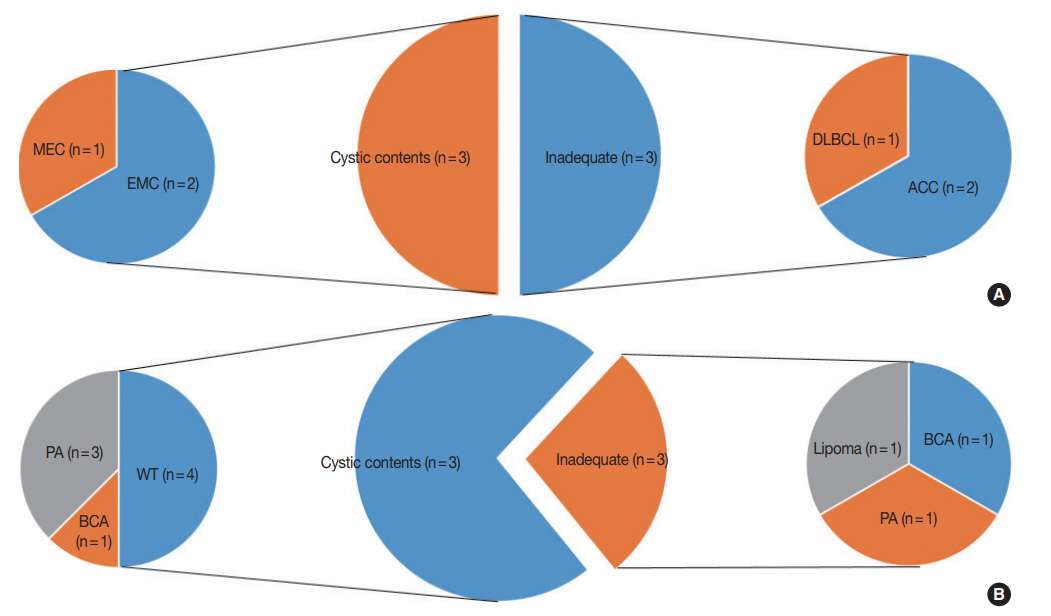
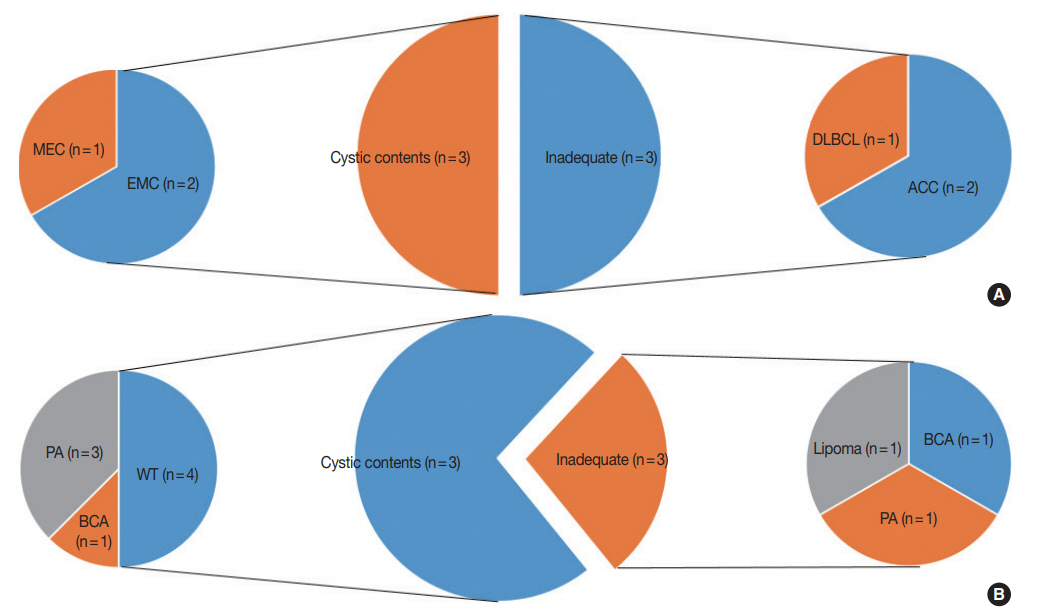
- This study included a total of 148 cases. The clinicopathologic characteristics of the patients are shown in Table 1. Our study population was made up of 64 (43.2%) male and 84 (56.8%) female patients, with a median age of 49 years (range, 11 to 85 years). FNA was performed predominantly from the parotid gland (n=120, 81.1%). Thirty-four cases (23.0%) were confirmed as M neoplasms by histological categorization. Their categorization according to the MSRSGC is shown in Fig. 1.
- Correlation between pathologic diagnosis and diagnosis based on MSRSGC categorization of FNA results
- The preoperative cytological diagnoses and histological follow-up results are listed in Table 2. There were 25 cases with category I FNAs: eight NN lesions, 11 BNs, and six M neoplasms. There were four cases of category II FNAs: two NN lesions and two BNs. Among the five cases with category III FNAs, three involved pleomorphic adenomas and the other two were one case each of metastatic breast cancer in the intraglandular lymph node and diffuse large B-cell lymphoma. Notably, our results showed that 97.5% (79/81) of category IV-A cases were BN. However, the FNAs of one mucoepidermoid carcinoma and one epithelial myoepithelial carcinoma were placed into category IV-A. Among the category IV-B cases, seven were BN and seven were M neoplasms. In contrast, all category V cases involved malignant tumors, half of which were epithelial myoepithelial carcinomas. Among the eight category VI FNA cases, seven involved malignant neoplasms, and one was an atypical pleomorphic adenoma.
- Further analysis of category I cases
- Of all FNA cases that were followed by surgical resection, category I cases accounted for 17% (25/148). Of the 25 category I cases, 22 were located in the parotid gland, which is significantly higher than the remaining three cases that involved the submandibular gland. The lesions diagnosed as category I in the submandibular gland included fibrocalcific nodules, IgG4-related disease, and chronic sialadenitis. There were six category I FNA cases with lesions diagnosed as malignant tumors, which included two acinic cell carcinomas, two epithelial myoepithelial carcinomas, one diffuse large B-cell lymphoma, and one mucoepidermoid carcinoma. (Fig. 1). Moreover, there were 11 category I FNA cases with lesions diagnosed as benign tumors, including four pleomorphic adenomas, four Warthin tumors, two basal cell adenomas, and one lipoma. Only cystic contents without cells were aspirated in four cases involving Warthin tumors and three cases involving pleomorphic adenomas (Fig. 1B).
- Risk stratification and comparisons with previous studies
- We found that the RON was 68.0% for ND, 50.0% for NN, 100% for AUS, 100% for BN, 93.3% for SUMP, 100% for SM, and 100% for M. The corresponding ROM values were 24.0% for ND, 0% for NN, 40.0% for AUS, 2.5% for BN, 46.7% for SUMP, 100% for SM, and 87.5% for M. A summary of the ROM values obtained in the current study and those proposed by the MSRSGC and other studies is shown in Table 3.
- Discrepant cases
- A discrepancy between FNA and pathological diagnoses was observed in six cases (Table 4). In the NN FNA group (two cases), one case was diagnosed as a Warthin tumor and the other as a sialolipoma. Among the two cases classified as BNs using the FNA samples, both were reported as malignant on resection (mucoepidermoid carcinoma and epithelial myoepithelial carcinoma). Moreover, among the cases classified as SUMPs, one was later diagnosed as a reactive lymph node (paracortical hyperplasia). Finally, among the cases categorized as malignant using FNA samples, one was diagnosed as atypical pleomorphic adenoma upon histological follow-up (Fig. 2). The diagnostic accuracy achieved using FNA samples was 95.8% (115/120 cases) for the parotid gland and 96.4% (27/28 cases) for the submandibular gland. Thus, the overall diagnostic accuracy using FNA samples was 95.9% (142/148 cases).
RESULTS
- In this study, we reclassified FNA cases from a single center using the MSRSGC and correlated the results with those obtained using pathological diagnoses. Most salivary gland FNA cases that completed surgical follow-up were benign (103 cases; 69.6%), which is similar to the incidence reported by other studies [14-17]. The diagnosis rate of ND at our institution was 23.5%, with 68.0% RON and 24.0% ROM. Notably, the frequency of ND diagnosis was higher than the 10% frequency set by the MSRSGC.
- The ROM of ND in our study was high compared to that of other studies [14-19]. Many factors are involved in an ND diagnosis, including aspiration technique, character of the lesion, method of specimen processing, and presence of artifacts from slide preparation [20]. Previous studies have suggested that rare, highly atypical cells could be placed in a category V if there is enough clinical suspicion, even if the number of cells to support this classification is insufficient [20].
- In this study, we divided category I cases into subtypes and examined their characteristics. Interestingly, 12.5% (4/32) of all Warthin tumors were placed in category I, and all four of these cases had only cystic contents. It is presumed that Warthin tumors could be accompanied by cystic degenerative changes in the center of the lesion. When category I was divided into two subtypes (inadequate and cystic contents only), there were no significant differences between the ROM values of the two subtypes (25% and 23.1%, respectively).
- The ROM values for the NN and BN categories in our study were 0% and 2.5%, respectively, while the RON values were 50.0% and 100%. The ROM values for the NN and BN categories were lower than the proposed ROM incidence of 10% and lower than the 5% ROM proposed by the MSRSGC. In comparison, the ROM values for the NN and BN categories in other studies ranged from 1.6%-17.4% and from 1.9%-7.3%, respectively (Table 3) [14-19]. In our study, Warthin tumors and sialolipoma cases were reported in the NN category, which is in contrast to the findings of studies by Rossi et al. [16], Viswanathan et al. [15], and Song et al. [14], in which B-cell lymphomas predominantly accounted for false-negative diagnoses. The increase in ROM in the BN category in our study was attributed to one case of low-grade mucoepidermoid carcinoma and one case of epithelial myoepithelial carcinoma, which exhibited a similar distribution to that observed in the study by Rossi et al. [16]. Moreover, in the study by Song et al., the increase in ROM in the BN category was attributed to three cases of carcinoma ex pleomorphic adenoma and one case of adenoid cystic carcinoma [14].
- According to the MSRSGC guidelines, cases in the AUS category should not exceed 10% of the total cases examined. In the current study, 3.8% of salivary gland FNAs were categorized as AUS, which is in accordance with this recommendation. The ROM for the AUS category was 40%, which is higher than the 20% proposed by the MSRSGC. However, one limitation of our study was that only five AUS FNAs were included, precluding precise analysis of ROM for this type. In comparison, other studies have reported an ROM ranging from 0-100%. Moreover, various entities were included in this category, with pleomorphic adenoma being the most common.
- The RON and ROM values for the SUMP category in our study were 93.3% and 46.7%, respectively, with pleomorphic adenoma and mucoepidermoid carcinoma being the most common benign and malignant diagnoses, respectively. The ROM was higher than the MSRSGC target rate and was similar to those reported in other studies (Song et al. [14] and Viswanathan et al. [15] reported ROMs of 46.6% and 34.2%, respectively). For the SM category, the RON and ROM values were 100% and 100%, respectively. Notably, the reported ROM for this category varies from institution to institution, ranging from 78.9%-100% [14-19]. This outcome is likely due to different institutional practices and disease populations, as well as differences in pathologist experience. Thus, this finding may represent a limitation of single-center studies. The RON and ROM values for the M category were 100% and 87.5%, respectively. The ROM in our study was slightly lower compared to that in the MSRSGC and other published studies [14-19]. In previous studies, squamous cell carcinoma was the most common malignant tumor in the M category [14-16]. However, the M category in our study consisted of only one case with squamous cell carcinoma; instead, mucoepidermoid carcinoma was the most frequently diagnosed malignancy. Compared to other studies, the limited sample size (eight cases) in our study may have led to both a lower ROM and greater differences in tumor type [14-19].
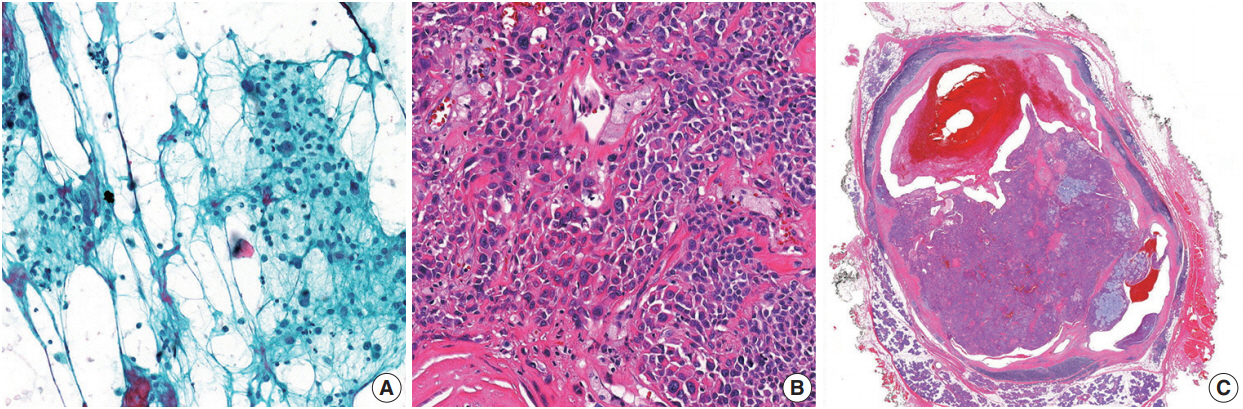
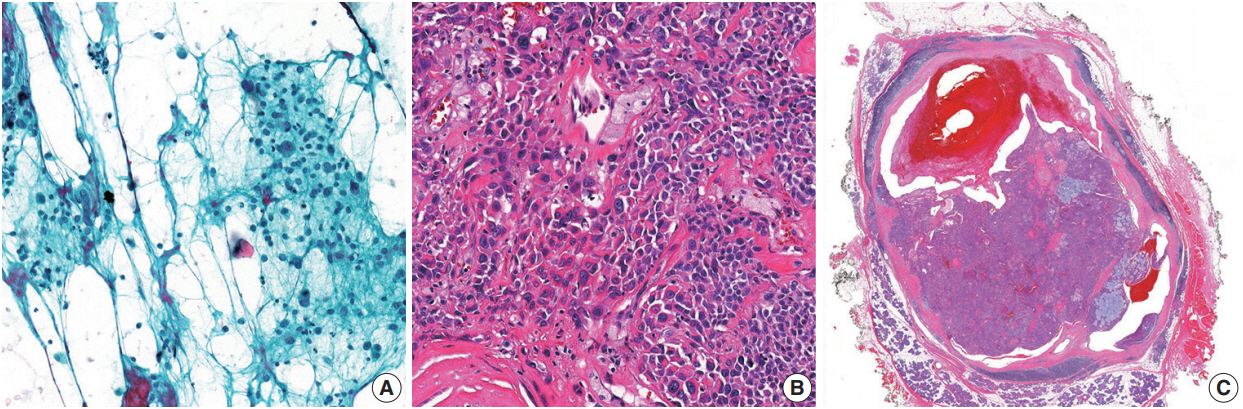
- In this study, there were four false-negative and two false positive cases. Contributing factors might have included sampling errors, inadequacy of technique, vagueness in interpretation, and underestimation of low-grade malignant tumors. In particular, mucoepidermoid carcinomas with cystic changes are difficult to diagnose due to a high incidence of failure to gain optimal material [21,22]. A limited number of mucoepidermoid carcinoma cases contains all three cell types (mucous, intermediate, and squamous cells) [23]. However, among malignant tumors, mucoepidermoid carcinoma could often be relatively straightforwardly assigned to the SM category when mucus cells are present. One of the false positive cases in the present study involved an atypical pleomorphic adenoma; the FNA specimen showed several clusters composed of markedly atypical cells in the degenerated background (Fig. 2). In the resected specimens, except for focal cytologic atypia, there were no features that indicated malignancy. Rohilla et al. [17] reported a similar false-positive case. Given this intriguing case, careful consideration of both the radiological findings and clinical assessments may help improve the predictive power of the MSRSGC.
- In conclusion, we confirmed that the ROM at our institute was similar to the proposed ROM. Thus, the MSRSGC appeared to be effective in facilitating communication between pathologists and clinicians and may lead to a more comprehensive understanding of cytological diagnoses and establishment of appropriate treatment strategies.
DISCUSSION
Ethics Statement
This retrospective study was approved by the Institutional Review Board of Gangnam Severance Hospital (approval No. 3-2019-0219) with a waiver of informed consent due to its retrospective nature.
Author contributions
Conceptualization: SWH. Data curation: JHP, YJC, JYL, SWH. Formal analysis: JHP, YJC. Investigation: JHP, YJC, JYL, SWH. Methodology: JHP, JYS, YJC, JYL, SWH. Project administration: SWH. Resources: JHP, JYS, YJC, JYL, SWH. Supervision: SWH. Validation: JHP, YJC, JYL, SWH. Visualization: JHP. Writing—original draft: JHP, SWH. Writing—review & editing: JHP, YJC, JYL, SWH.
Conflicts of Interest
SWH, a contributing editor of the Journal of Pathology and Translational Medicine, was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Funding Statement
No funding to declare.
| ND | NN | AUS | BN | SUMP | SM | M | |
|---|---|---|---|---|---|---|---|
| ROM proposed by the MSRSGC | 25.0 | 10.0 | 20.0 | < 5 | 35.0 | 60.0 | 90.0 |
| Current study | 24.0 | 0 | 40.0 | 2.5 | 46.7 | 100 | 87.5 |
| Rossi et al. [16] | 17.0 | 16.0 | 53.0 | 6a | 79.0 | 100 | |
| Song et al. [14] | 17.8 | 14.3 | 30.6 | 2.2 | 46.6 | 78.9 | 98.8 |
| Viswanathan et al. [15] | 6.7 | 7.1 | 38.9 | 5.0 | 34.2 | 92.9 | 92.3 |
| Thiryayi et al. [19] | 8.5 | 1.6 | 0 | 1.9 | 26.7 | 100 | 100 |
| Rohilla et al. [17] | 0 | 17.4 | 100 | 7.3 | 50.0 | - | 96.0 |
| Park et al. [18] | 19.5 | 6.9 | 0 | 2.4 | 26.2 | 83.3 | 100 |
ND, non-diagnostic; NN, non-neoplastic; AUS, atypia of undetermined significance; BN, benign neoplasm; SUMP, salivary gland neoplasm of uncertain malignant potential; SM, suspicious for malignancy; M, malignant; ROM, risk of malignancy; MSRSGC, Milan System for Reporting Salivary Gland Cytopathology.
aCases in the BN and SUMP categories were calculated together in this study.
- 1. Layfield LJ, Gopez E, Hirschowitz S. Cost efficiency analysis for fine-needle aspiration in the workup of parotid and submandibular gland nodules. Diagn Cytopathol 2006; 34: 734-8. ArticlePubMed
- 2. Mairembam P, Jay A, Beale T, et al. Salivary gland FNA cytology: role as a triage tool and an approach to pitfalls in cytomorphology. Cytopathology 2016; 27: 91-6. ArticlePubMed
- 3. Wang H, Fundakowski C, Khurana JS, Jhala N. Fine-needle aspiration biopsy of salivary gland lesions. Arch Pathol Lab Med 2015; 139: 1491-7. ArticlePubMedPDF
- 4. Seethala RR, Griffith CC. Molecular pathology: predictive, prognostic, and diagnostic markers in salivary gland tumors. Surg Pathol Clin 2016; 9: 339-52. PubMed
- 5. Pusztaszeri MP, Garcia JJ, Faquin WC. Salivary gland FNA: new markers and new opportunities for improved diagnosis. Cancer Cytopathol 2016; 124: 307-16. PubMed
- 6. Pusztaszeri MP, Faquin WC. Update in salivary gland cytopathology: Recent molecular advances and diagnostic applications. Semin Diagn Pathol 2015; 32: 264-74. ArticlePubMed
- 7. Goyal S, Sharma S, Diwaker P. Diagnostic role and limitations of FNAC in oral and jaw swellings. Diagn Cytopathol 2015; 43: 810-8. ArticlePubMed
- 8. Schindler S, Nayar R, Dutra J, Bedrossian CW. Diagnostic challenges in aspiration cytology of the salivary glands. Semin Diagn Pathol 2001; 18: 124-46. PubMed
- 9. Ashraf A, Shaikh AS, Kamal F, Sarfraz R, Bukhari MH. Diagnostic reliability of FNAC for salivary gland swellings: a comparative study. Diagn Cytopathol 2010; 38: 499-504. ArticlePubMed
- 10. Jain R, Gupta R, Kudesia M, Singh S. Fine needle aspiration cytology in diagnosis of salivary gland lesions: a study with histologic comparison. Cytojournal 2013; 10: 5.PubMedPMC
- 11. Colella G, Cannavale R, Flamminio F, Foschini MP. Fine-needle aspiration cytology of salivary gland lesions: a systematic review. J Oral Maxillofac Surg 2010; 68: 2146-53. ArticlePubMed
- 12. Rossi ED, Baloch Z, Pusztaszeri M, Faquin WC. The Milan System for Reporting Salivary Gland Cytopathology (MSRSGC): an ASCIAC-sponsored system for reporting salivary gland fine-needle aspiration. J Am Soc Cytopathol 2018; 7: 111-8. ArticlePubMed
- 13. Rossi ED, Faquin WC, Baloch Z, et al. The Milan System for Reporting Salivary Gland Cytopathology: analysis and suggestions of initial survey. Cancer Cytopathol 2017; 125: 757-66. ArticlePubMedPDF
- 14. Song SJ, Shafique K, Wong LQ, LiVolsi VA, Montone KT, Baloch Z. The utility of the Milan System as a risk stratification tool for salivary gland fine needle aspiration cytology specimens. Cytopathology 2019; 30: 91-8. ArticlePubMedPDF
- 15. Viswanathan K, Sung S, Scognamiglio T, Yang GC, Siddiqui MT, Rao RA. The role of the Milan System for Reporting Salivary Gland Cytopathology: a 5-year institutional experience. Cancer Cytopathol 2018; 126: 541-51. ArticlePubMedPDF
- 16. Rossi ED, Wong LQ, Bizzarro T, et al. The impact of FNAC in the management of salivary gland lesions: institutional experiences leading to a risk-based classification scheme. Cancer Cytopathol 2016; 124: 388-96. ArticlePubMed
- 17. Rohilla M, Singh P, Rajwanshi A, et al. Three-year cytohistological correlation of salivary gland FNA cytology at a tertiary center with the application of the Milan system for risk stratification. Cancer Cytopathol 2017; 125: 767-75. ArticlePubMedPDF
- 18. Park W, Bae H, Park MH, et al. Risk of high-grade malignancy in parotid gland tumors as classified by the Milan System for Reporting Salivary Gland Cytopathology. J Oral Pathol Med 2019; 48: 222-31. ArticlePubMedPDF
- 19. Thiryayi SA, Low YX, Shelton D, Narine N, Slater D, Rana DN. A retrospective 3-year study of salivary gland FNAC with categorisation using the Milan reporting system. Cytopathology 2018; 29: 343-8. ArticlePubMedPDF
- 20. Faquin WC, Rossi ED, Baloch Z, et al. The Milan System for Reporting Salivary Gland Cytopathology. New York: Springer, 2018.
- 21. Edwards PC, Wasserman P. Evaluation of cystic salivary gland lesions by fine needle aspiration: an analysis of 21 cases. Acta Cytol 2005; 49: 489-94. PubMed
- 22. Frable MA, Frable WJ. Fine-needle aspiration biopsy of salivary glands. Laryngoscope 1991; 101: 245-9. ArticlePubMed
- 23. Joseph TP, Joseph CP, Jayalakshmy PS, Poothiode U. Diagnostic challenges in cytology of mucoepidermoid carcinoma: report of 6 cases with histopathological correlation. J Cytol 2015; 32: 21-4. ArticlePubMedPMC
REFERENCES
Figure & Data
References
Citations

- The Impact of Lesion-Specific and Sampling-Related Factors on Success of Salivary Gland Fine-Needle Aspiration Cytology
Marcel Mayer, Mohammad Marwan Alfarra, Kathrin Möllenhoff, Marianne Engels, Christoph Arolt, Alexander Quaas, Philipp Wolber, Louis Jansen, Lisa Nachtsheim, Maria Grosheva, Jens Peter Klussmann, Sami Shabli
Head and Neck Pathology.2025;[Epub] CrossRef - The Myriad Spectrum of Salivary Gland Lesions: Cytohistological Correlation on Fine Needle Aspiration Cytology, Core Needle Biopsy, and Resections in a 5‐Year Single Institutional Experience of North India
Zachariah Chowdhury, Pallavi Majumdar, Sumeet Narain, Komal Lamba
Diagnostic Cytopathology.2025; 53(8): 391. CrossRef - Diagnostic Performance of the Milan System for Reporting Salivary Gland Cytopathology and a Proposed Algorithm for Fine-Needle Aspiration Cytology of Salivary Gland Lesions
Norihide Mochizuki, Hirotaka Fujita, Takuma Tajiri, Masataka Ueda, Makiko Kurata, Chie Inomoto, Tomoko Sugiyama, Daisuke Maki, Shuichi Shiraishi, Tomohisa Machida, Hitoshi Ito, Yohei Masugi, Naoya Nakamura
Acta Cytologica.2025; 69(4): 324. CrossRef - A study of fine needle aspiration cytology and histopathology correlation of salivary gland neoplasms in a tertiary care hospital: an observational study
Asima Malik, Ahlam Mushtaq, Salma Bhat, Suhail Naik
International Journal of Contemporary Pediatrics.2025; 13(1): 23. CrossRef - The Milan system for reporting salivary gland cytopathology – Assessment of utility and the risk of malignancy
Annu E. Prakash, Renu Sukumaran, Nileena Nayak, K. Lakshmi, Anitha Mathews, Jayasree Kattoor
Indian Journal of Cancer.2024; 61(3): 575. CrossRef - Salivary gland fine-needle aspiration biopsy: quality assurance results from a tertiary cancer center
Fanni Ratzon, Dominique L. Feliciano, Nora Katabi, Bin Xu, Oscar Lin, Xiao-Jun Wei
Journal of the American Society of Cytopathology.2023; 12(3): 206. CrossRef - Cytohistological correlation and risk stratification of salivary gland lesions using the Milan System for Reporting Salivary Gland Cytopathology: A tertiary care centre experience
Tarun Kumar, Prerna Tewari, Jitendra Singh Nigam, Shreekant Bharti, Surabhi, Ruchi Sinha, Punam Prasad Bhadani
Cytopathology.2023; 34(3): 225. CrossRef - Assessment of Risk of Malignancy of Fine-needle Aspiration Cytology in Salivary Gland Lesions Using the Milan System for Reporting Salivary Gland Cytopathology Categorization: A Systematic Review and Meta-analysis
Amit Kumar, Subhash Chandra, Bishnupati Singh, Swati Sharma, Ankita Tandon, Ajoy Kumar Shahi
The Journal of Contemporary Dental Practice.2023; 23(10): 1039. CrossRef - Milan Sınıflandırma Sistemi’ne Göre Değerlendirilen Tükürük Bezi İnce İğne Aspirasyon Sitolojilerinin Histopatolojik Tanı Uyumu
Özlem SARAYDAROĞLU, Selin YİRMİBEŞ
Uludağ Üniversitesi Tıp Fakültesi Dergisi.2023; 49(3): 285. CrossRef - Milan system for reporting salivary gland cytopathology: Adoption and outcomes in a community setting
Samih J. Nassif, Ali R. Sasani, Garrey T. Faller, Jennifer L. Harb, Jagdish K. Dhingra
Head & Neck.2022; 44(6): 1462. CrossRef - Nondiagnostic salivary gland FNAs are associated with decreased risk of malignancy compared with “all‐comer” patients: Analysis of the Milan System for Reporting Salivary Gland Cytopathology with a focus on Milan I: Nondiagnostic
Shu K. Lui, Troy Tenney, Patrick C. Mullane, Kartik Viswanathan, Daniel J. Lubin
Cancer Cytopathology.2022; 130(10): 800. CrossRef - Application of the Milan System for Reporting Salivary Gland Cytopathology: A systematic review and meta‐analysis
Zhaoyang Wang, Huan Zhao, Huiqin Guo, Changming An
Cancer Cytopathology.2022; 130(11): 849. CrossRef - Multiplexed single‐cell analysis of FNA allows accurate diagnosis of salivary gland tumors
Juhyun Oh, Tae Yeon Yoo, Talia M. Saal, Lisa Tsay, William C. Faquin, Jonathan C.T. Carlson, Daniel G. Deschler, Sara I. Pai, Ralph Weissleder
Cancer Cytopathology.2022; 130(8): 581. CrossRef - Cytologic analysis of vitreous fluids: A retrospective review of our 24 years of experience
Gabriel L. Collins, Elizabeth W. Hubbard, Christopher T. Clark, Lisa D. Duncan, Laurentia Nodit
Diagnostic Cytopathology.2021; 49(10): 1122. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure


Fig. 1.
Fig. 2.
| Characteristic | No. (%) |
|---|---|
| Sex | |
| Male | 64 (43.2) |
| Female | 84 (56.8) |
| Age (yr), mean ± SD (range) | 50.0 ± 15.1 (11–85) |
| Location | |
| Parotid | 120 (81.1) |
| Submandibular | 28 (18.9) |
| MSRSGC category | |
| I | 26 (16.9) |
| II | 4 (2.7) |
| III | 5 (3.4) |
| IV-A | 81 (54.7) |
| IV-B | 15 (10.1) |
| V | 10 (6.8) |
| VI | 8 (5.4) |
| Pathologic diagnosis category | |
| Non-tumor lesion | 11 (7.4) |
| Benign neoplasm | 103 (69.6) |
| Malignant neoplasm | 34 (23.0) |
| MSRSGC category | Pathological diagnosis |
||
|---|---|---|---|
| Non-neoplastic (n = 11) | Benign neoplasm (n = 103) | Malignant neoplasm (n = 34) | |
| I (n = 25) | Lymphoepithelial cyst (n = 4) | WT (n = 4) | ACC (n = 2) |
| IgG4-related disease (n = 2) | PA (n = 4) | EMC (n = 2) | |
| Chronic sialadenitis (n = 1) | Lipoma (n = 1) | DLBCL (n = 1) | |
| Fibrocalcific nodule (n = 1) | BCA (n = 2) | MEC (n = 1) | |
| II (n = 4) | Reactive lymph node (n = 1) | WT (n = 1) | None |
| Epidermal cyst (n = 1) | Sialolipoma (n = 1) | ||
| III (n = 5) | None | PA (n = 3) | Metastatic carcinoma (n = 1) |
| DLBCL (n = 1) | |||
| IV-A (n = 81) | None | PA (n = 48) | MEC (n = 1) |
| WT (n = 27) | EMC (n = 1) | ||
| Oncocytoma (n = 1) | |||
| BCA (n = 1) | |||
| Myoepithelioma (n = 1) | |||
| Atypical PA (n = 1) | |||
| IV-B (n = 15) | Reactive lymph node (n = 1) | PA (n = 4) | MEC (n = 3) |
| Oncocytoma (n = 1) | EMC (n = 2) | ||
| Myoepithelioma (n = 1) | ACC (n = 1) | ||
| Hemangioma (n = 1) | Carcinoma ex PA (n = 1) | ||
| V (n = 10) | None | None | MEC (n = 5) |
| AdCC (n = 2) | |||
| ACC (n = 1) | |||
| DLBCL (n = 1) | |||
| Sqcc (n = 1) | |||
| VI (n = 8) | None | Atypical PA (n = 1) | MEC (n = 3) |
| ACC (n = 2) | |||
| Adenocarcinoma, NOS (n = 1) | |||
| Metastatic melanoma (n = 1) | |||
| ND | NN | AUS | BN | SUMP | SM | M | |
|---|---|---|---|---|---|---|---|
| ROM proposed by the MSRSGC | 25.0 | 10.0 | 20.0 | < 5 | 35.0 | 60.0 | 90.0 |
| Current study | 24.0 | 0 | 40.0 | 2.5 | 46.7 | 100 | 87.5 |
| Rossi et al. [16] | 17.0 | 16.0 | 53.0 | 6 |
79.0 | 100 | |
| Song et al. [14] | 17.8 | 14.3 | 30.6 | 2.2 | 46.6 | 78.9 | 98.8 |
| Viswanathan et al. [15] | 6.7 | 7.1 | 38.9 | 5.0 | 34.2 | 92.9 | 92.3 |
| Thiryayi et al. [19] | 8.5 | 1.6 | 0 | 1.9 | 26.7 | 100 | 100 |
| Rohilla et al. [17] | 0 | 17.4 | 100 | 7.3 | 50.0 | - | 96.0 |
| Park et al. [18] | 19.5 | 6.9 | 0 | 2.4 | 26.2 | 83.3 | 100 |
| No. | Type of discrepancy | MSRSGC category | Final pathologic diagnosis |
|---|---|---|---|
| 1 | False-negative | IV-A | Mucoepidermoid carcinoma, low-grade |
| 2 | False-negative | IV-A | Epithelial myoepithelial carcinoma |
| 3 | False-negative | II | Warthin tumor |
| 4 | False-negative | II | Sialolipoma |
| 5 | False positive | VI | Atypical pleomorphic adenoma |
| 6 | False positive | IV-B | Reactive lymph node |
MSRSGC, Milan System for Reporting Salivary Gland Cytopathology.
WT, Warthin tumor; ACC, acinic cell carcinoma; PA, pleomorphic adenoma; EMC, epithelial myoepithelial carcinoma; DLBCL, diffuse large B-cell lymphoma; BCA, basal cell adenoma; MEC, mucoepidermoid carcinoma; AdCC, adenoid cystic carcinoma; Sqcc, squamous cell carcinoma; NOS, not otherwise specified.
ND, non-diagnostic; NN, non-neoplastic; AUS, atypia of undetermined significance; BN, benign neoplasm; SUMP, salivary gland neoplasm of uncertain malignant potential; SM, suspicious for malignancy; M, malignant; ROM, risk of malignancy; MSRSGC, Milan System for Reporting Salivary Gland Cytopathology. Cases in the BN and SUMP categories were calculated together in this study.
FNA, fine-needle aspiration; MSRSGC, Milan System for Reporting Salivary Gland Cytopathology.

 E-submission
E-submission