Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 46(3); 2012 > Article
-
Case Report
Tumor Budding and Recurrence in Submucosal Invasive Colorectal Cancers of Favorable Histology: Case Reports of Two Early Colorectal Cancers with Advanced Recurrences - Heae Surng Park, Hee Jin Chang1,2, Ji Won Park1, Byung Chang Kim1, Dae Kyung Sohn1, Chang Won Hong1, Ji-Yeon Baek1, Sun Young Kim1, Hyo Seong Choi1, Jae Hwan Oh1
-
Korean Journal of Pathology 2012;46(3):272-277.
DOI: https://doi.org/10.4132/KoreanJPathol.2012.46.3.272
Published online: June 22, 2012
Department of Pathology, Seoul National University College of Medicine, Seoul, Korea.
1Center for Colorectal Cancer, National Cancer Center, Goyang, Korea.
2Department of Pathology, Research Institute and Hospital, National Cancer Center, Goyang, Korea.
- Corresponding Author: Hee Jin Chang, M.D. Center for Colorectal Cancer and Department of Pathology, Research Institute and Hospital, National Cancer Center, 323 Ilsan-ro, Ilsandong-gu, Goyang 410-769, Korea. Tel: +82-31-920-1741, Fax: +82-31-920-1369, heejincmd@yahoo.com
• Received: November 1, 2010 • Revised: January 5, 2011 • Accepted: January 6, 2011
© 2012 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations
Citations to this article as recorded by 

- Estudio de factores histológicos predictivos de metástasis ganglionar locorregional en adenocarcinoma colorrectal mínimamente invasivo pT1
Isidro Machado, Miriam Valera-Alberni, Fernando Martínez de Juan, José A. López-Guerrero, Alfonso García Fadrique, Julia Cruz, Carmen Martínez Lapiedra, Fernanda Maia de Alcantara, Ricardo Yaya, Jorge Campos, Carlos Fernández-Martos, Rafael Estevan
Gastroenterología y Hepatología.2016; 39(1): 1. CrossRef - Histological factors predicting loco-regional lymph node metastasis in early invasive colorectal adenocarcinoma pT1
Isidro Machado, Miriam Valera-Alberni, Fernando Martínez de Juan, José A. López-Guerrero, Alfonso García Fadrique, Julia Cruz, Carmen Martínez Lapiedra, Fernanda Maia de Alcantara, Ricardo Yaya, Jorge Campos, Carlos Fernández-Martos, Rafael Estevan
Gastroenterología y Hepatología (English Edition).2016; 39(1): 1. CrossRef - Tumor budding in the clinical management of colon and rectal cancer
Viktor H Koelzer, Inti Zlobec, Alessandro Lugli
Colorectal Cancer.2014; 3(4): 387. CrossRef
Tumor Budding and Recurrence in Submucosal Invasive Colorectal Cancers of Favorable Histology: Case Reports of Two Early Colorectal Cancers with Advanced Recurrences






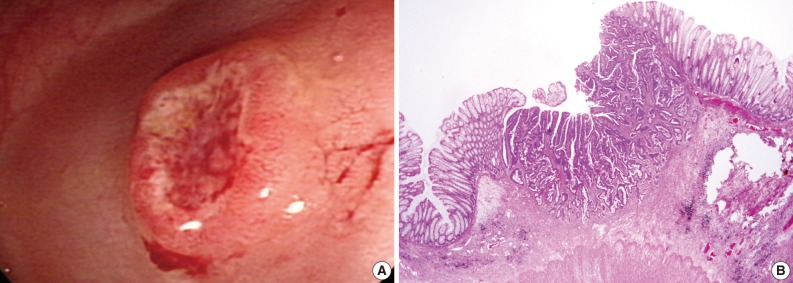
Fig. 1 Endoscopic and histologic findings of primary tumor in case 1. (A) Colonoscopic examination shows subpedunculated mass in the rectum with an ill-defined boundary. (B) Pathologic examination reveals a rectal cancer infiltrating the submucosa, with an adenoma component at the periphery.
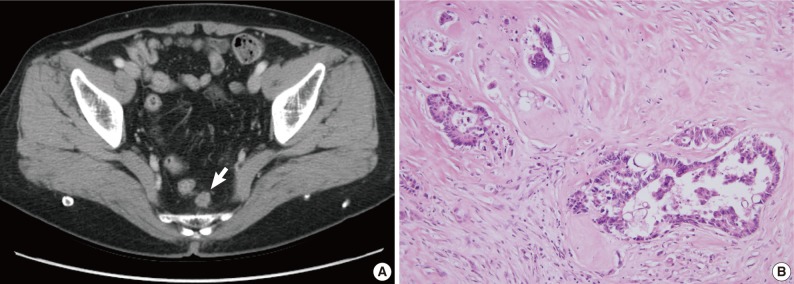
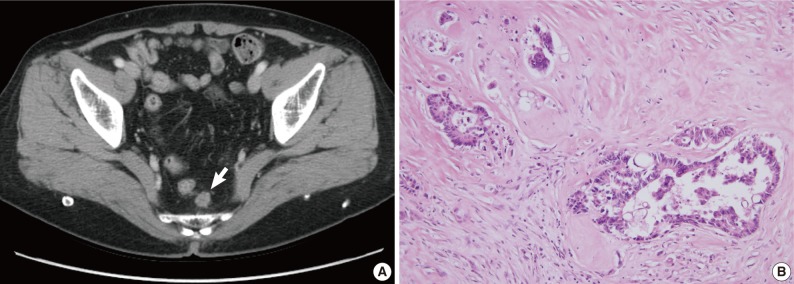
Fig. 2 Radiologic and histologic findings of recurrent tumor in case 1. (A) A newly appeared soft tissue mass (arrow) in the perirectal tissue is detected upon computed tomographic imaging. (B) A perirectal mass shows an adenocarcinoma with intraluminal necrosis.
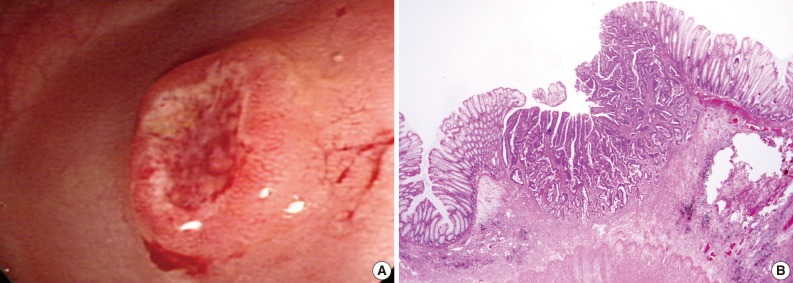
Fig. 3 Endoscopic and histologic findings of primary tumor in case 2. (A) Colonoscopic examination shows an elevated tumor with central depression in the sigmoid colon. (B) Pathologic examination reveals a colon cancer invading the submucosa.
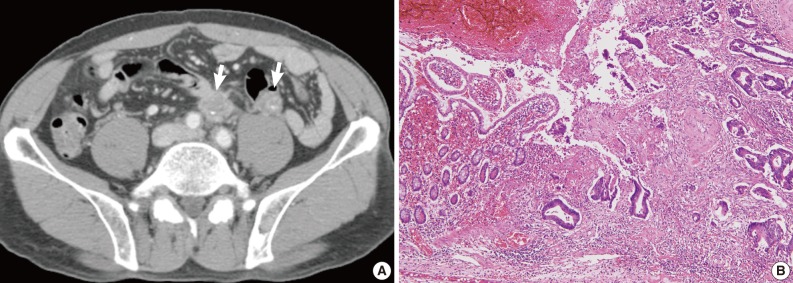
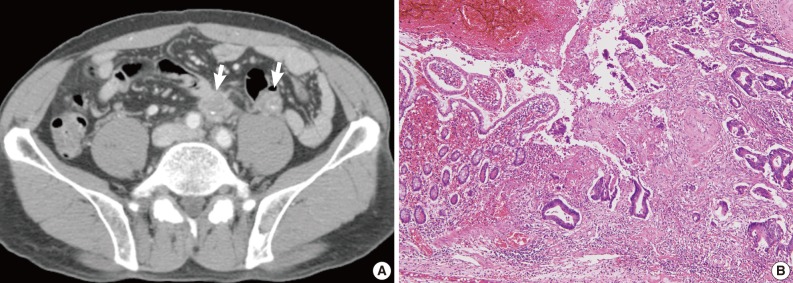
Fig. 4 Radiologic and histologic findings of recurrent tumor in case 2. (A) Multiple metastatic lesions (arrows) in the para-aortic lymph node and mesentery are identified on computed tomographic imaging. (B) Adenocarcinoma involves the small intestinal mucosa, with similar histological findings to the original tumor (Fig. 2B).
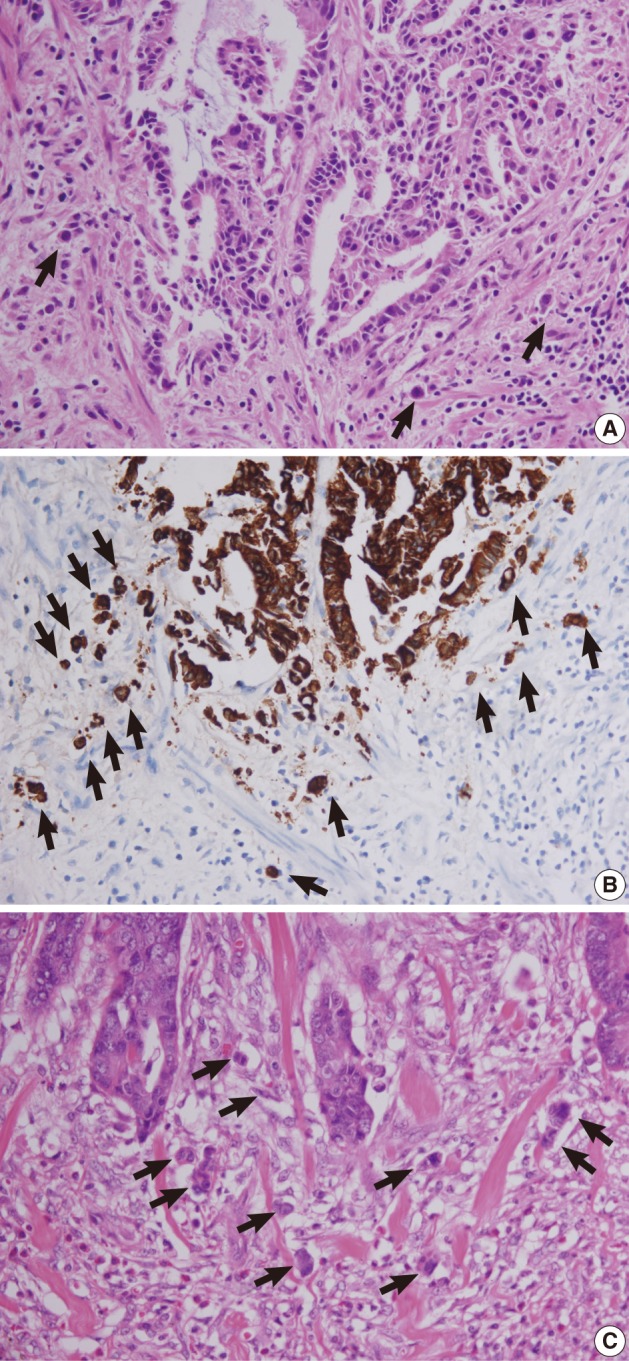
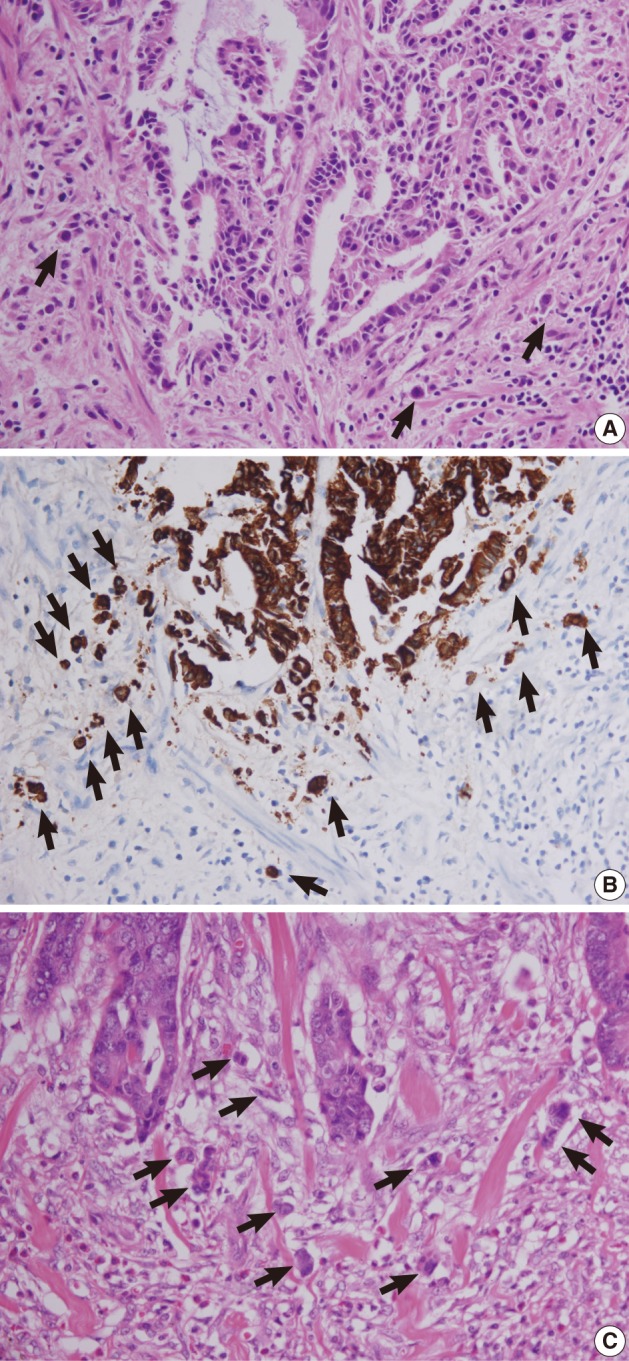
Fig. 5 Tumor buds at the invasive front of primary tumors (arrows). (A, B) In case 1, subtle budding cells (A) around the tumor margins are highlighted by immunohistochemistry using antibody to cytokeratin (B). (C) In case 2, frequent tumor budding is observed in a high power field.
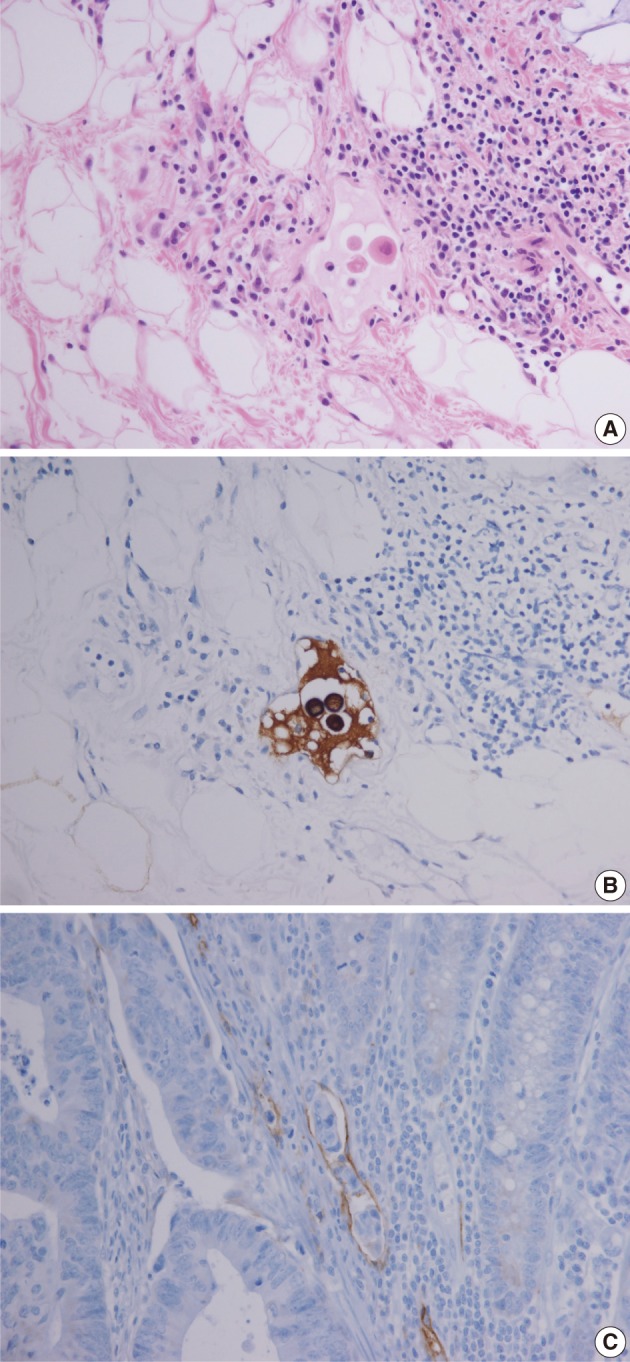
Fig. 6 Lymphatic invasion in the primary tumors. (A, B) In case 1, several atypical cells are observed in the lymphatic channel (A) and proven to be carcinoma cells positive for cytokeratin (B). (C) In case 2, endolymphatic tumor emboli at the tumor border are confirmed by antibody to D2-40.
Fig. 1
Fig. 2
Fig. 3
Fig. 4
Fig. 5
Fig. 6
Tumor Budding and Recurrence in Submucosal Invasive Colorectal Cancers of Favorable Histology: Case Reports of Two Early Colorectal Cancers with Advanced Recurrences

 E-submission
E-submission

 PubReader
PubReader Cite this Article
Cite this Article