Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 47(1); 2013 > Article
-
Original Article
Microsatellite Instability Status in Gastric Cancer: A Reappraisal of Its Clinical Significance and Relationship with Mucin Phenotypes - Joo-Yeun Kim,, Na Ri Shin,, Ahrong Kim, Hyun-Jeong Lee, Won-young Park, Jee-Yeon Kim, Chang-Hun Lee, Gi-Young Huh1, Do Youn Park
-
Korean Journal of Pathology 2013;47(1):28-35.
DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.1.28
Published online: February 25, 2013
Department of Pathology, Biomedical Research Institute, Pusan National University Hospital, Pusan National University School of Medicine, Busan, Korea.
1Department of Forensic Medicine, Biomedical Research Institute, Pusan National University Hospital, Pusan National University School of Medicine, Busan, Korea.
- Corresponding Author: Do Youn Park, M.D. Department of Pathology, Pusan National University Hospital, Pusan National University School of Medicine, 179 Gudeok-ro, Seo-gu, Busan 602-739, Korea. Tel: +82-51-240-7717, Fax: +82-51-256-0788, pdy220@pusan.ac.kr
- *Joo-Yeun Kim and Na Ri Shin contributed equally to this work.
© 2013 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations

- Intestinal Subtype as a Biomarker of Response to Neoadjuvant Immunochemotherapy in Locally Advanced Gastric Adenocarcinoma: Insights from a Prospective Phase II Trial
Lei Wang, Mengting Sun, Jinyang Li, Linghong Wan, Yuting Tan, Shuoran Tian, Yongying Hou, Linyu Wu, Ziyi Peng, Xiao Hu, Qihua Zhang, Zening Huang, Mengyi Han, Shiyin Peng, Yuwei Pan, Yuanfeng Ren, Mengsi Zhang, Dongfeng Chen, Qin Liu, Xianfeng Li, Zhong-y
Clinical Cancer Research.2025; 31(1): 74. CrossRef - How do I treat dMMR/MSI gastro-oesophageal adenocarcinoma in 2025? A position paper from the EORTC-GITCG gastro-esophageal task force
Christelle de la Fouchardière, Antonella Cammarota, Magali Svrcek, Maria Alsina, Tania Fleitas-Kanonnikoff, Radka Lordick Obermannová, Anna Dorothea Wagner, Dominic Yap Wei Ting, Diana Enea, Angelica Petrillo, Elizabeth C. Smyth
Cancer Treatment Reviews.2025; 134: 102890. CrossRef - T-bet+CD8+ T cells govern anti-PD-1 responses in microsatellite-stable gastric cancers
Shiying Tang, Xiaofang Che, Jinyan Wang, Ce Li, Xin He, Kezuo Hou, Xiaojie Zhang, Jia Guo, Bowen Yang, Danni Li, Lili Cao, Xiujuan Qu, Zhenning Wang, Yunpeng Liu
Nature Communications.2025;[Epub] CrossRef - Prediction of a Panel of Programmed Cell Death Protein-1 (PD-1) Inhibitor–Sensitive Biomarkers Using Multiphase Computed Tomography Imaging Textural Features: Retrospective Cohort Analysis
Shiqi Wang, Na Chai, Jingji Xu, Pengfei Yu, Luguang Huang, Quan Wang, Zhifeng Zhao, Bin Yang, Jiangpeng Wei, Xiangjie Wang, Gang Ji, Minwen Zheng
JMIR Cancer.2025; 11: e67379. CrossRef - Isolated tumor cell clusters (ITC) in lymph nodes and PD-L1 expression on tumor-associated immune cells are prognostic factors for microsatellite instable-high gastric cancers
Menghan Cui, Yangli Zhou, Yin Han, Nannan Chen, Min Zhao, Yan Wang, Fengxia He
Translational Oncology.2025; 59: 102465. CrossRef - Microsatellite Instability and BAT-26 Marker Expression in a Mexican Prostate Cancer Population with Different Gleason Scores
Ana K. Flores-Islas, Manuel A. Rico-Méndez, Marisol Godínez-Rubí, Martha Arisbeth Villanueva-Pérez, Erick Sierra-Díaz, Ana Laura Pereira-Suárez, Saul A. Beltrán-Ontiveros, Perla Y. Gutiérrez-Arzapalo, José M. Moreno-Ortiz, Adrián Ramírez-de-Arellano
Diseases.2025; 13(7): 202. CrossRef - Non–Pure Intestinal Phenotype as an Indicator of Progression in Sporadic Nonampullary Duodenal Adenomas: A Multicenter Retrospective Cohort Study
Ryotaro Uema, Yoshito Hayashi, Masato Komori, Narihiro Shibukawa, Noriko Hayashi, Masayoshi Horimoto, Takuya Yamada, Masashi Yamamoto, Satoshi Hiyama, Kazuo Kinoshita, Hideharu Ogiyama, Shinjiro Yamaguchi, Satoshi Egawa, Takashi Kanesaka, Minoru Kato, Shu
Clinical and Translational Gastroenterology.2024; 15(1): e00649. CrossRef - Intratumoral and peritumoral CT-based radiomics for predicting the microsatellite instability in gastric cancer
Xingchi Chen, Zijian Zhuang, Lin Pen, Jing Xue, Haitao Zhu, Lirong Zhang, Dongqing Wang
Abdominal Radiology.2024; 49(5): 1363. CrossRef - The tumor immune composition of mismatch repair deficient and Epstein-Barr virus-positive gastric cancer: A systematic review
J. Bos, T.S. Groen-van Schooten, C.P. Brugman, F.S. Jamaludin, H.W.M. van Laarhoven, S. Derks
Cancer Treatment Reviews.2024; 127: 102737. CrossRef - Potent therapeutic strategy in gastric cancer with microsatellite instability-high and/or deficient mismatch repair
Akira Ooki, Hiroki Osumi, Koichiro Yoshino, Kensei Yamaguchi
Gastric Cancer.2024; 27(5): 907. CrossRef - The mechanism of RGS5 regulating gastric cancer mismatch repair protein
Zhenwei Yang, Ranran Zhang, Jialong Liu, Sufang Tian, Hailin Zhang, Lingxiu Zeng, Yangyang Zhang, Liping Gao, Meng Wang, Wenqing Shan, Jing Liu
Molecular Carcinogenesis.2024; 63(9): 1750. CrossRef - Prognostic significance of microsatellite instability in patients with resectable gastric cancer
Marina Alessandra Pereira, Marcus Fernando Kodama Pertille Ramos, Leonardo Cardili, André Roncon Dias, Venancio Avancini Ferreira Alves, Evandro Sobroza de Mello, Ulysses Ribeiro
Journal of Gastrointestinal Surgery.2024; 28(10): 1687. CrossRef - Access to radiotherapy in improving gastric cancer care quality and equality
Minmin Wang, Kepei Huang, Xiaohan Fan, Jia Wang, Yinzi Jin, Zhi-Jie Zheng
Communications Medicine.2024;[Epub] CrossRef - Deep learning captures selective features for discrimination of microsatellite instability from pathologic tissue slides of gastric cancer
Sung Hak Lee, Yujin Lee, Hyun‐Jong Jang
International Journal of Cancer.2023; 152(2): 298. CrossRef - Novel Biomarkers of Gastric Cancer: Current Research and Future Perspectives
Yasushi Sato, Koichi Okamoto, Yutaka Kawano, Akinari Kasai, Tomoyuki Kawaguchi, Tamotsu Sagawa, Masahiro Sogabe, Hiroshi Miyamoto, Tetsuji Takayama
Journal of Clinical Medicine.2023; 12(14): 4646. CrossRef - The results of treatment for resectable gastric cancer with microsatellite instability
H. Sun, S. N. Nered, A. A. Tryakin, E. V. Artamonova, A. E. Kalinin, V. E. Bugaev, A. M. Stroganova, N. S. Besova, P. P. Arkhiri, V. I. Marshall, R. Sh. Abdulaeva, I. S. Stilidi
Pelvic Surgery and Oncology.2023; 13(2): 17. CrossRef - Heterogeneity and Adjuvant Therapeutic Approaches in MSI-H/dMMR Resectable Gastric Cancer: Emerging Trends in Immunotherapy
Hui Wu, Wenyuan Ma, Congfa Jiang, Ning Li, Xin Xu, Yongfeng Ding, Haiping Jiang
Annals of Surgical Oncology.2023; 30(13): 8572. CrossRef - Dual-layer spectral-detector CT for predicting microsatellite instability status and prognosis in locally advanced gastric cancer
Yongjian Zhu, Peng Wang, Bingzhi Wang, Zhichao Jiang, Ying Li, Jun Jiang, Yuxin Zhong, Liyan Xue, Liming Jiang
Insights into Imaging.2023;[Epub] CrossRef - Concordance between microsatellite instability testing and immunohistochemistry for mismatch repair proteins and efficient screening of mismatch repair deficient gastric cancer
Gou Yamamoto, Tetsuya Ito, Okihide Suzuki, Nao Kamae, Miho Kakuta, Akemi Takahashi, Katsuya Iuchi, Tomio Arai, Hideyuki Ishida, Kiwamu Akagi
Oncology Letters.2023;[Epub] CrossRef - Low incidence of microsatellite instability in gastric cancers and its association with the clinicopathological characteristics: a comparative study
Fateme Fooladi Talari, Ali Bozorg, Sirous Zeinali, Mohammadreza Zali, Zhale Mohsenifar, Hamid Asadzadeh Aghdaei, Kaveh Baghaei
Scientific Reports.2023;[Epub] CrossRef - Mutational separation and clinical outcomes of TP53 and CDH1 in gastric cancer
He-Li Liu, Huan Peng, Chang-Hao Huang, Hai-Yan Zhou, Jie Ge
World Journal of Gastrointestinal Surgery.2023; 15(12): 2855. CrossRef - Genomic and Immunologic Markers of Intrinsic Resistance to Pembrolizumab Monotherapy in Microsatellite Instability-High Gastric Cancer: Observations from a Prospective Phase II Study
Haibo Qiu
Global Medical Genetics.2022; 09(02): 060. CrossRef - Clinicopathological features of PD-L1 protein expression, EBV positivity, and MSI status in patients with advanced gastric and esophagogastric junction adenocarcinoma in Japan
Tsutomu Yoshida, Go Ogura, Mikiko Tanabe, Takuo Hayashi, Chiho Ohbayashi, Mizutomo Azuma, Chikara Kunisaki, Yoichi Akazawa, Soji Ozawa, Sohei Matsumoto, Takayoshi Suzuki, Akira Mitoro, Tetsu Fukunaga, Akiko Shimizu, Go Fujimoto, Takashi Yao
Cancer Biology & Therapy.2022; 23(1): 191. CrossRef - Development of Tissue-Agnostic Treatments for Patients with Cancer
Steven Lemery, Lola Fashoyin-Aje, Leigh Marcus, Sandra Casak, Julie Schneider, Marc Theoret, Paul Kluetz, Richard Pazdur, Julia A. Beaver
Annual Review of Cancer Biology.2022; 6(1): 147. CrossRef - A multicenter study on the preoperative prediction of gastric cancer microsatellite instability status based on computed tomography radiomics
Xiuqun Liang, Yinbo Wu, Ying Liu, Danping Yu, Chencui Huang, Zhi Li
Abdominal Radiology.2022; 47(6): 2036. CrossRef - Combination of AKT1 and CDH1 mutations predicts primary resistance to immunotherapy in dMMR/MSI-H gastrointestinal cancer
Zhenghang Wang, Qi Zhang, Changsong Qi, Yuezong Bai, Feilong Zhao, Hui Chen, Zhongwu Li, Xicheng Wang, Mifen Chen, Jifang Gong, Zhi Peng, Xiaotian Zhang, Jinping Cai, Shiqing Chen, Xiaochen Zhao, Lin Shen, Jian Li
Journal for ImmunoTherapy of Cancer.2022; 10(6): e004703. CrossRef - Eldest gastric cancer patient with high microsatellite instability responding to pembrolizumab
Akinobu Wakasugi, Akinori Sasaki, Risa Okamoto, Yasuaki Motomura
International Cancer Conference Journal.2022; 12(1): 59. CrossRef - Baseline lesion number as an efficacy predictive and independent prognostic factor and its joint utility with TMB for PD-1 inhibitor treatment in advanced gastric cancer
Xiao-Li Wei, Jian-Ying Xu, De-Shen Wang, Dong-Liang Chen, Chao Ren, Jia-Ning Li, Feng Wang, Feng-Hua Wang, Rui-Hua Xu
Therapeutic Advances in Medical Oncology.2021;[Epub] CrossRef - Clinical and morphological portrait of tumors with microsatellite instability
A. A. Musaelyan, V. D. Nazarov, A. S. Budnikova, S. V. Lapin, S. L. Vorobyev, V. L. Emanuel, A. A. Zakharenko, S. V. Orlov
Advances in Molecular Oncology.2021; 8(2): 52. CrossRef - How to Best Exploit Immunotherapeutics in Advanced Gastric Cancer: Between Biomarkers and Novel Cell-Based Approaches
Michele Ghidini, Angelica Petrillo, Andrea Botticelli, Dario Trapani, Alessandro Parisi, Anna La Salvia, Elham Sajjadi, Roberto Piciotti, Nicola Fusco, Shelize Khakoo
Journal of Clinical Medicine.2021; 10(7): 1412. CrossRef - Microsatellite instability in Gastric Cancer: Between lights and shadows
Elisabetta Puliga, Simona Corso, Filippo Pietrantonio, Silvia Giordano
Cancer Treatment Reviews.2021; 95: 102175. CrossRef - Impact of microsatellite status on negative lymph node count and prognostic relevance after curative gastrectomy
Zhenghao Cai, Junjun Ma, Shuchun Li, Abe Fingerhut, Jing Sun, Lu Zang, Chao Yan, Wentao Liu, Zhenggang Zhu, Minhua Zheng
Journal of Surgical Oncology.2021;[Epub] CrossRef - A greater lymph node yield is required during pathological examination in microsatellite instability-high gastric cancer
Zhenghao Cai, Haiqin Song, Abe Fingerhut, Jing Sun, Junjun Ma, Luyang Zhang, Shuchun Li, Chaoran Yu, Minhua Zheng, Lu Zang
BMC Cancer.2021;[Epub] CrossRef - Determinants of Response and Intrinsic Resistance to PD-1 Blockade in Microsatellite Instability–High Gastric Cancer
Minsuk Kwon, Minae An, Samuel J. Klempner, Hyuk Lee, Kyoung-Mee Kim, Jason K. Sa, Hee Jin Cho, Jung Yong Hong, Taehyang Lee, Yang Won Min, Tae Jun Kim, Byung-Hoon Min, Woong-Yang Park, Won Ki Kang, Kyu-Tae Kim, Seung Tae Kim, Jeeyun Lee
Cancer Discovery.2021; 11(9): 2168. CrossRef - Advanced Gastric Cancer: Current Treatment Landscape and a Future Outlook for Sequential and Personalized Guide: Swiss Expert Statement Article
Alexander R. Siebenhüner, Sara De Dosso, Daniel Helbling, Christoforos Astaras, Petr Szturz, Peter Moosmann, Stefanie Pederiva, Thomas Winder, Philippe Von Burg, Markus Borner
Oncology Research and Treatment.2021; 44(9): 485. CrossRef - High homogeneity of mismatch repair deficiency in advanced prostate cancer
Christoph Fraune, Ronald Simon, Doris Höflmayer, Katharina Möller, David Dum, Franziska Büscheck, Claudia Hube-Magg, Georgia Makrypidi-Fraune, Martina Kluth, Andrea Hinsch, Eike Burandt, Till Sebastian Clauditz, Waldemar Wilczak, Guido Sauter, Stefan Steu
Virchows Archiv.2020; 476(5): 745. CrossRef - High homogeneity of MMR deficiency in ovarian cancer
Christoph Fraune, Janina Rosebrock, Ronald Simon, Claudia Hube-Magg, Georgia Makrypidi-Fraune, Martina Kluth, Franziska Büscheck, Doris Höflmayer, Barbara Schmalfeldt, Volkmar Müller, Linn Wölber, Isabell Witzel, Peter Paluchowski, Christian Wilke, Uwe He
Gynecologic Oncology.2020; 156(3): 669. CrossRef - Molecular Classification of Gastric Cancer among Alaska Native People
Holly Martinson, Dominic Mallari, Christine Richter, Tsung-Teh Wu, James Tiesinga, Steven Alberts, Matthew Olnes
Cancers.2020; 12(1): 198. CrossRef - Tumor immune response and immunotherapy in gastric cancer
Yoonjin Kwak, An Na Seo, Hee Eun Lee, Hye Seung Lee
Journal of Pathology and Translational Medicine.2020; 54(1): 20. CrossRef - MMR deficiency in urothelial carcinoma of the bladder presents with temporal and spatial homogeneity throughout the tumor mass
Christoph Fraune, Ronald Simon, Claudia Hube-Magg, Georgia Makrypidi-Fraune, Christian Kähler, Martina Kluth, Doris Höflmayer, Franziska Büscheck, David Dum, Andreas M. Luebke, Eike Burandt, Till Sebastian Clauditz, Waldemar Wilczak, Guido Sauter, Stefan
Urologic Oncology: Seminars and Original Investigations.2020; 38(5): 488. CrossRef - MMR Deficiency is Homogeneous in Pancreatic Carcinoma and Associated with High Density of Cd8-Positive Lymphocytes
Christoph Fraune, Eike Burandt, Ronald Simon, Claudia Hube-Magg, Georgia Makrypidi-Fraune, Martina Kluth, Franziska Büscheck, Doris Höflmayer, Niclas Ch. Blessin, Tim Mandelkow, Wenchao Li, Daniel Perez, Jakob R. Izbicki, Waldemar Wilczak, Guido Sauter, J
Annals of Surgical Oncology.2020; 27(10): 3997. CrossRef - CD73's Potential as an Immunotherapy Target in Gastrointestinal Cancers
Jerry B. Harvey, Luan H. Phan, Oscar E. Villarreal, Jessica L. Bowser
Frontiers in Immunology.2020;[Epub] CrossRef - Tumor copy-number alterations predict response to immune-checkpoint-blockade in gastrointestinal cancer
Zhihao Lu, Huan Chen, Shuang Li, Jifang Gong, Jian Li, Jianling Zou, Lihong Wu, Jianing Yu, Wenbo Han, Huaibo Sun, Xi Jiao, Xiaotian Zhang, Zhi Peng, Ming Lu, Zhenghang Wang, Henghui Zhang, Lin Shen
Journal for ImmunoTherapy of Cancer.2020; 8(2): e000374. CrossRef - Protein expression-based classification of gastric cancer by immunohistochemistry of tissue microarray
Chong Zhao, Zhiqiang Feng, Hongzhen He, Dan Zang, Hong Du, Hongli Huang, Yanlei Du, Jie He, Yongjian Zhou, Yuqiang Nie, Girijesh Kumar Patel
PLOS ONE.2020; 15(10): e0238836. CrossRef - Clinicopathologic Characteristics and Long-Term Outcome of Gastric Cancer Patients with Family History: Seven-Year Follow-Up Study for Korean Health Check-Up Subjects
Jooyoung Lee, Su Jin Chung, Ji Min Choi, Yoo Min Han, Joo Sung Kim, Greger Lindberg
Gastroenterology Research and Practice.2020; 2020: 1. CrossRef - Implication of expression of MMR proteins and clinicopathological characteristics in gastric cancer
Renu Verma, Puja Sakhuja, Ritu Srivastava, Prakash Chand Sharma
Asia-Pacific Journal of Oncology.2020; : 1. CrossRef - Prognostic significance of microsatellite‐instability in gastric and gastroesophageal junction cancer patients undergoing neoadjuvant chemotherapy
Georg Martin Haag, Elena Czink, Aysel Ahadova, Thomas Schmidt, Leila Sisic, Susanne Blank, Ulrike Heger, Leonidas Apostolidis, Anne Katrin Berger, Christoph Springfeld, Felix Lasitschka, Dirk Jäger, Magnus von Knebel Doeberitz, Matthias Kloor
International Journal of Cancer.2019; 144(7): 1697. CrossRef - Serological Markers Associated With Response to Immune Checkpoint Blockade in Metastatic Gastrointestinal Tract Cancer
Zhihao Lu, Jianling Zou, Ying Hu, Shuang Li, Tao Zhou, Jifang Gong, Jian Li, Xiaotian Zhang, Jun Zhou, Ming Lu, Xicheng Wang, Zhi Peng, Changsong Qi, Yanyan Li, Jie Li, Yan Li, Jianyin Zou, Xiao Du, Henghui Zhang, Lin Shen
JAMA Network Open.2019; 2(7): e197621. CrossRef - Assessing molecular subtypes of gastric cancer: microsatellite unstable and Epstein-Barr virus subtypes. Methods for detection and clinical and pathological implications
Carolina Martinez-Ciarpaglini, Tania Fleitas-Kanonnikoff, Valentina Gambardella, Marta Llorca, Cristina Mongort, Regina Mengual, Gema Nieto, Lara Navarro, Marisol Huerta, Susana Rosello, Desamparados Roda, Noelia Tarazona, Samuel Navarro, Gloria Ribas, An
ESMO Open.2019; 4(3): e000470. CrossRef - The role of pembrolizumab in the treatment of PD-L1 expressing gastric and gastroesophageal junction adenocarcinoma
Gagandeep Brar, Manish A. Shah
Therapeutic Advances in Gastroenterology.2019;[Epub] CrossRef - Novel Biomarkers for Prediction of Response to Preoperative Systemic Therapies in Gastric Cancer
Alessandro Cavaliere, Valeria Merz, Simona Casalino, Camilla Zecchetto, Francesca Simionato, Hayley Louise Salt, Serena Contarelli, Raffaela Santoro, Davide Melisi
Journal of Gastric Cancer.2019; 19(4): 375. CrossRef - MICROSATELLITE INSTABILITY AND GASTRIC CARCINOMA. REVIEW OF THELITERATURE
D. L. Rotin, O. V. Paklina, I. O. Tin’kova, D. N. Grekov
Russian Journal of Biotherapy.2019; 18(4): 17. CrossRef - Meta-analysis of microsatellite instability in relation to clinicopathological characteristics and overall survival in gastric cancer
K Polom, L Marano, D Marrelli, R De Luca, G Roviello, V Savelli, P Tan, F Roviello
Journal of British Surgery.2018; 105(3): 159. CrossRef - Gastric poorly cohesive carcinoma: a correlative study of mutational signatures and prognostic significance based on histopathological subtypes
Chae H Kwon, Young K Kim, Sojeong Lee, Ahrong Kim, Hye J Park, Yuri Choi, Yeo J Won, Do Y Park, Gregory Y Lauwers
Histopathology.2018; 72(4): 556. CrossRef - Microsatellite instability in gastric cancer: molecular bases, clinical perspectives, and new treatment approaches
Margherita Ratti, Andrea Lampis, Jens C. Hahne, Rodolfo Passalacqua, Nicola Valeri
Cellular and Molecular Life Sciences.2018; 75(22): 4151. CrossRef - High-throughput Protein and mRNA Expression–based Classification of Gastric Cancers Can Identify Clinically Distinct Subtypes, Concordant With Recent Molecular Classifications
Sangjeong Ahn, So-Jeong Lee, Yonugkeum Kim, Ahrong Kim, Nari Shin, Kyung Un Choi, Chang-Hun Lee, Gi Yeong Huh, Kyong-Mee Kim, Namrata Setia, Gregory Y. Lauwers, Do Youn Park
American Journal of Surgical Pathology.2017; 41(1): 106. CrossRef - Molecular Testing for Gastrointestinal Cancer
Hye Seung Lee, Woo Ho Kim, Yoonjin Kwak, Jiwon Koh, Jeong Mo Bae, Kyoung-Mee Kim, Mee Soo Chang, Hye Seung Han, Joon Mee Kim, Hwal Woong Kim, Hee Kyung Chang, Young Hee Choi, Ji Y. Park, Mi Jin Gu, Min Jin Lhee, Jung Yeon Kim, Hee Sung Kim, Mee-Yon Cho
Journal of Pathology and Translational Medicine.2017; 51(2): 103. CrossRef - Molecular testing of gastrointestinal tumours
Matthew Evans, Matthew Smith, Brendan O'Sullivan, Philippe Taniere
Diagnostic Histopathology.2017; 23(10): 442. CrossRef - Gastric Carcinomas With Lymphoid Stroma
Raul S Gonzalez, Justin M M Cates, Frank Revetta, Loralee A McMahon, Kay Washington
American Journal of Clinical Pathology.2017; 148(6): 477. CrossRef - Meta-Analysis of Prognostic Role of Ki-67 Labeling Index in Gastric Carcinoma
Jung-Soo Pyo, Nae Yu Kim
The International Journal of Biological Markers.2017; 32(4): 447. CrossRef - Tissue-Agnostic Drug Development
Keith T. Flaherty, Dung T. Le, Steven Lemery
American Society of Clinical Oncology Educational Book.2017; (37): 222. CrossRef - Programmed death ligand-1 and MET co-expression is a poor prognostic factor in gastric cancers after resection
Mi Jung Kwon, Kab-Choong Kim, Eun Sook Nam, Seong Jin Cho, Hye-Rim Park, Soo Kee Min, Jinwon Seo, Ji-Young Choe, Hye Kyung Lee, Ho Suk Kang, Kyueng-Whan Min
Oncotarget.2017; 8(47): 82399. CrossRef - Hypermutation and microsatellite instability in gastrointestinal cancers
Kizuki Yuza, Masayuki Nagahashi, Satoshi Watanabe, Kazuaki Takabe, Toshifumi Wakai
Oncotarget.2017; 8(67): 112103. CrossRef - The Emerging Role of Immunotherapy in Gastric and Esophageal Adenocarcinoma
Bruno Bockorny, Eirini Pectasides
Future Oncology.2016; 12(15): 1833. CrossRef - Expression of Mismatch Repair Proteins in Early and Advanced Gastric Cancer in Poland
Katarzyna Karpińska-Kaczmarczyk, Magdalena Lewandowska, Małgorzata Ławniczak, Andrzej Białek, Elżbieta Urasińska
Medical Science Monitor.2016; 22: 2886. CrossRef - Immunotherapy for Gastroesophageal Cancer
Emily Goode, Elizabeth Smyth
Journal of Clinical Medicine.2016; 5(10): 84. CrossRef - Lauren classification and individualized chemotherapy in gastric cancer
JUNLI MA, HONG SHEN, LINDA KAPESA, SHAN ZENG
Oncology Letters.2016; 11(5): 2959. CrossRef - High-risk and low-risk gastric cancer areas in Italy and its association with microsatellite instability
Karol Polom, Daniele Marrelli, Valeria Pascale, Giandomenico Roviello, Costantino Voglino, Henry Rho, Carla Vindigni, Mario Marini, Raffaele Macchiarelli, Franco Roviello
Journal of Cancer Research and Clinical Oncology.2016; 142(8): 1817. CrossRef - MUC2 Expression Is Correlated with Tumor Differentiation and Inhibits Tumor Invasion in Gastric Carcinomas: A Systematic Review and Meta-analysis
Jung-Soo Pyo, Jin Hee Sohn, Guhyun Kang, Dong-Hoon Kim, Kyungeun Kim, In-Gu Do, Dong Hyun Kim
Journal of Pathology and Translational Medicine.2015; 49(3): 249. CrossRef - Correlation between microsatellite instability-high phenotype and occult lymph node metastasis in gastric carcinoma
Jiwoon Choi, Soo Kyung Nam, Do Joong Park, Hwal Woong Kim, Hyung-Ho Kim, Woo Ho Kim, Hye Seung Lee
APMIS.2015; 123(3): 215. CrossRef - Clinicopathologic and molecular features associated with patient age in gastric cancer
Ji Yeon Seo, Eun Hyo Jin, Hyun Jin Jo, Hyuk Yoon, Cheol Min Shin, Young Soo Park, Nayoung Kim, Hyun Chae Jung, Dong Ho Lee
World Journal of Gastroenterology.2015; 21(22): 6905. CrossRef - Molecular classification of gastric cancer: Towards a pathway-driven targeted therapy
Ismael Riquelme, Kathleen Saavedra, Jaime A. Espinoza, Helga Weber, Patricia García, Bruno Nervi, Marcelo Garrido, Alejandro H. Corvalán, Juan Carlos Roa, Carolina Bizama
Oncotarget.2015; 6(28): 24750. CrossRef - A phylogenetic model for understanding the effect of gene duplication on cancer progression
Qin Ma, Jaxk H. Reeves, David A. Liberles, Lili Yu, Zheng Chang, Jing Zhao, Juan Cui, Ying Xu, Liang Liu
Nucleic Acids Research.2014; 42(5): 2870. CrossRef - The analysis of microsatellite instability in extracolonic gastrointestinal malignancy
Andrew S. Williams, Weei-Yuarn Huang
Pathology.2013; 45(6): 540. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure



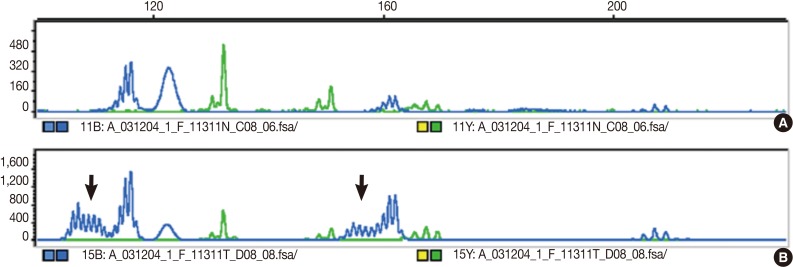
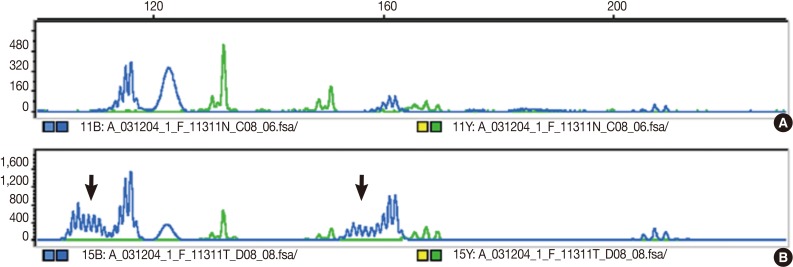
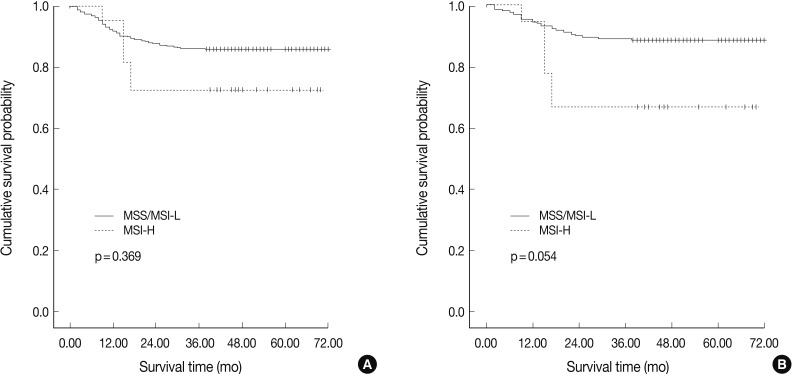
Fig. 1
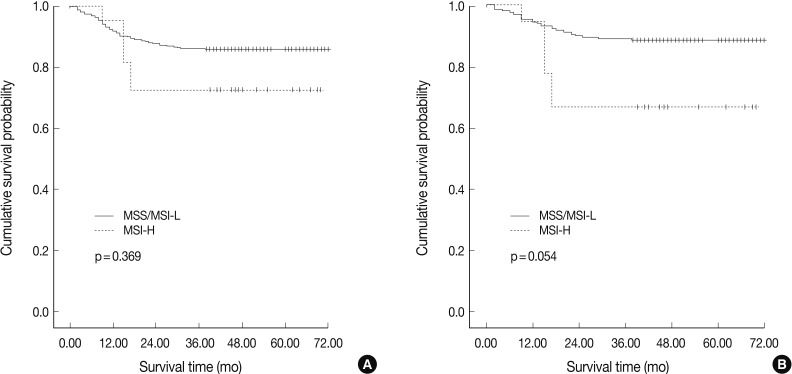
Fig. 2
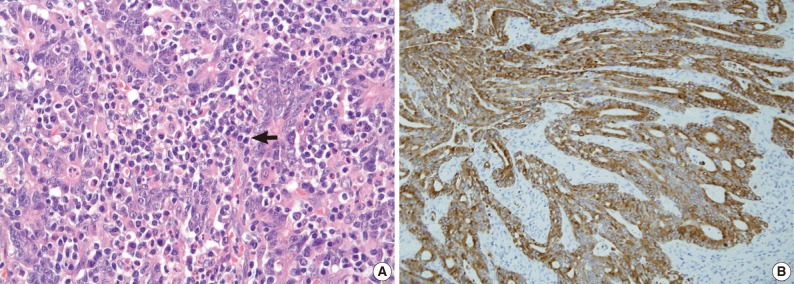
Fig. 3
| Primary antibody (clone) | Source | Dilution |
|---|---|---|
| MUC2 (CLH2) | Novocastra Laboratories, Newcastle, UK | 1 : 500 |
| MUC5AC (CLH5) | Novocastra Laboratories, Newcastle, UK | 1 : 500 |
| MUC6 (Ccp58) | Novocastra Laboratories, Newcastle, UK | 1 : 500 |
| CD10 (56C6) | Novocastra Laboratories, Newcastle, UK | 1 : 100 |
| No. of cases | MSI status |
p-value | ||
|---|---|---|---|---|
| MSS/MSI-L | MSI-H | |||
| Age (yr) | 58.59 ± 11.22 | 64 ± 11.00 | 0.010 | |
| Gender | ||||
| Male | 288 | 270 (93.8) | 18 (6.2) | 0.419 |
| Female | 126 | 121 (96.0) | 5 (4.0) | |
| Tumor size (cm) | 3.77 ± 2.64 | 5.18 ± 3.01 | 0.014 | |
| Location | ||||
| Upper/Middle | 195 | 188 (96.4) | 7 (3.6) | 0.140 |
| Lower | 219 | 204 (94.4) | 15 (5.6) | |
| Invasion depth (+) | ||||
| T1 | 225 | 219 (97.3) | 6 (2.7) | 0.009 |
| T2 | 50 | 47 (94.0) | 3 (6.0) | |
| T3 | 86 | 78 (90.7) | 8 (9.3) | |
| T4 | 53 | 48 (90.6) | 5 (9.4) | |
| Gross type | ||||
| Elevated | 105 | 96 (91.4) | 9 (8.6) | 0.042 |
| Flat/Depressed | 180 | 176 (97.8) | 4 (2.2) | |
| Excavated | 129 | 120 (93.0) | 9 (7.0) | |
| Histologic type | ||||
| Intestinal | 237 | 219 (92.4) | 18 (7.6) | 0.028 |
| Diffuse | 162 | 158 (98.1) | 4 (1.9) | |
| Mixed | 15 | 14 (93.3) | 1 (6.7) | |
| Lymphovascular emboli | ||||
| Negative | 261 | 252 (96.6) | 9 (3.4) | 0.027 |
| Positive | 153 | 140 (91.5) | 13 (8.5) | |
| Perineural invasion | ||||
| Negative | 280 | 270 (96.4) | 10 (3.6) | 0.022 |
| Positive | 134 | 122 (91.0) | 12 (9.0) | |
| Lymph node metastasis | ||||
| Negative | 246 | 236 (95.9) | 10 (4.1) | 0.170 |
| Positive | 168 | 156 (92.9) | 12 (7.1) | |
| Variables | B | SE | HR (95% CI) | p-value |
|---|---|---|---|---|
| Age (≤ 59 yr vs >59 yr) | -0.213 | 0.335 | 0.808 (0.419-1.588) | 0.525 |
| Depth (EGC vs AGC) | -0.516 | 0.400 | 0.597 (0.273-1.308) | 0.198 |
| Site (upper and middle vs lower) | 0.580 | 0.380 | 1.785 (0.847-3.761) | 0.128 |
| LN metastasis ([+] vs [-]) | -1.140 | 0.398 | 0.320 (0.147-0.698) | 0.004 |
| MSI (MSI-H vs MSS/MSI-L) | -0.582 | 0.451 | 0.559 (0.231-1.353) | 0.197 |
| No. of cases | MSI status |
p-value | ||
|---|---|---|---|---|
| MSS/MSI-L | MSI-H | |||
| Tumor necrosis (%) | ||||
| < 10 | 388 | 370 (95.4) | 18 (4.6) | 0.041 |
| ≥ 10 | 26 | 22 (84.6) | 4 (15.4) | |
| Crohn’s-like reaction | ||||
| Absent | 208 | 199 (95.7) | 9 (4.3) | 0.368 |
| Present | 206 | 193 (93.7) | 13 (6.3) | |
| Extracellular mucin (%) | ||||
| < 10 | 366 | 345 (94.3) | 21 (5.7) | 0.289 |
| ≥ 10 | 48 | 47 (97.9) | 1 (2.1) | |
| Tumor infiltrating lymphocytes | ||||
| < 2/HPF | 337 | 327 (97.0) | 10 (3.0) | < 0.001 |
| ≥ 2/HPF | 77 | 65 (84.4) | 12 (15.6) | |
| Growth pattern | ||||
| Expanding+mixed | 179 | 162 (90.5) | 17 (9.5) | 0.038 |
| Infiltrative | 103 | 100 (97.1) | 3 (2.9) | |
| No. of cases | MSI status |
p-value | ||
|---|---|---|---|---|
| MSS/MSI-L | MSI-H | |||
| MUC2 (%) | ||||
| < 10 | 267 | 254 (95.1) | 13 (4.9) | 0.586 |
| ≥ 10 | 147 | 138 (93.9) | 9 (6.1) | |
| MUC5AC (%) | ||||
| < 10 | 135 | 131 (97.0) | 4 (3.0) | 0.138 |
| ≥ 10 | 279 | 261 (93.5) | 18 (6.5) | |
| MUC6 (%) | ||||
| < 10 | 228 | 221 (96.9) | 7 (3.1) | 0.024 |
| ≥ 10 | 186 | 170 (91.9) | 16 (8.1) | |
| CD10 (%) | ||||
| < 10 | 329 | 309 (93.9) | 20 (6.1) | 0.275 |
| ≥ 10 | 85 | 83 (97.6) | 2 (2.4) | |
| Mucin phenotype | ||||
| GCGP | 248 | 230 (92.7) | 18 (7.3) | 0.031 |
| GCIP+null | 168 | 162 (97.6) | 4 (2.4) | |
| Variables | B | SE | OR (95% CI) | p-value |
|---|---|---|---|---|
| Tumor necrosis ( ≥ 10% vs < 10%) | 1.415 | 0.657 | 4.118 (1.135-14.937) | 0.031 |
| TILs ( ≥ 2/HPF vs < 2/HPF) | 1.877 | 0.472 | 6.535 (2.591-16.486) | <0.001 |
| Lauren classification (intestinal+mixed vs diffuse) | 1.597 | 0.647 | 4.938 (1.388-17.566) | 0.014 |
| Mucin phenotypes (GC-GP vs GC-IP+null) | 1.356 | 0.583 | 3.881 (1.238-12.170) | 0.020 |
| Authors (yr) | n | Markers | Methods | MSI-H (%) | Survival |
|---|---|---|---|---|---|
| Present study | 414 | BAT25, BAT26, D5S346, D2S123, D17S250 | Fluorescence | 5.6 | MSI-H have poor survival in intestinal type gastric cancer |
| An et al. [25] (2012) | 1,990 | BAT25, BAT26, D5S346, D2S123, D17S250 | Fluorescence | 8.5 | No correlation |
| Oki et al. [24] (2009) | 240 | D2S123, D5S107, D10S197, D11S904, D13S175 | Fluorescence | 9.4 | No correlation |
| Seo et al. [12] (2009) | 328 | BAT25, BAT26, D5S346, D2S123, D17S250 | Fluorescence | 8.2 | No correlation |
| Falchetti et al. [6] (2008) | 159 | BAT25, BAT26, D1S104, D2S123,D3S1611, D5S107, D17S261, D18S342 | Fluorescence | 17.0 | MSI-H have good survival in gastric cancer |
| Beghelli et al. [7] (2006) | 510 | BAT25, BAT26 | Fluorescence | 16 | MSI-H have good survival in gastric cancer |
| An et al. [13] (2006) | 83 | BAT25, BAT26, D5S346, D2S123, D17S250 | Fluorescence | 19 | No correlation |
| Lee et al. [5] (2002) | 327 | BAT25, BAT26 | Fluorescence | 9.5 | MSI-H have good survival in advanced gastric cancer |
| Yamamoto et al. [4] (1999) | 205 | BAT25, AP△3, D1S158, D8S199, D5S421 | Radiolabelled | 14 | MSI-H have good survival in advanced gastric cancer |
| Wirtz et al. [14] (1998) | 126 | BAT25, BAT26, D2S119, D2S123, D5S107, D5S346, D10S197, D11S904, D17S261, D18S34 | Radiolabelled | 12.8 | No correlation |
MSI, microsatellite instability; MSS, microsatellite stable; MSI-L, low-level MSI; MSI-H, high-level MSI. Between T1 vs. T2+T3+T4; Between intestinal type+mixed type vs. diffuse type.
B, coefficient; SE, standard error; HR, hazard ratio; CI, confidence interval; EGC, early gastric cancer; AGC, advanced gastric cancer; LN, lymph node; MSI, microsatellite instability; MSI-H, high-level MSI; MSS, microsatellite stable; MSI-L, low-level MSI.
MSI, microsatellite instability; MSS, microsatellite stable; MSI-L, low-level MSI; MSI-H, high-level MSI; HPF, high power filed.
MSI, microsatellite instability; MSS, microsatellite stable; MSI-L, low-level MSI; MSI-H, high-level MSI; GCGP, gastric cancer with gastric mucin predominant type; GCIP, gastric cancer with intestinal mucin predominant type.
The clinicopathologic factors for MSI-H gastric cancers are analyzed by binary logistic regression analysis (backward, stepwise). MSI-H, high-level microsatellite instability; B, coefficient; SE, standard error; OR, odds ratio; CI, confidence interval; TIL, tumor-infiltrating lymphocytes; HPF, high power field; GC-GP, gastric cancer with gastric mucin predominant type; GC-IP, gastric cancer with intestinal mucin predominant type.
MSI-H, high-level microsatellite instability.

 E-submission
E-submission