Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 47(1); 2013 > Article
-
Case Study
Epithelioid Trophoblastic Tumor: Clinicopathologic and Immunohistochemical Analysis of Three Cases - Woo Jung Sung1,2, Hyeong Chan Shin1, Min-Kyung Kim2, Mi Jin Kim1
-
Korean Journal of Pathology 2013;47(1):67-73.
DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.1.67
Published online: February 25, 2013
1Department of Pathology, Yeungnam University College of Medicine, Daegu, Korea.
2Department of Pathology, Catholic University of Daegu School of Medicine, Daegu, Korea.
- Corresponding Author: Mi Jin Kim, M.D. Department of Pathology, Yeungnam University Collage of Medicine, 170 Hyeonchung-ro, Nam-gu, Daegu 705-717, Korea. Tel: +82-53-620-3334, Fax: +82-53-622-8432, mjkap@ynu.ac.kr
© 2013 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- Epithelioid trophoblastic tumor is an unusual type of trophoblastic tumor. Here we report on the clinicopathologic and immunohistochemical features of three cases of epithelioid trophoblastic tumor. All three patients were of reproductive age and presented with vaginal bleeding and mild elevation of human chorionic gonadotropin (hCG). All patients underwent a hysterectomy. The tumors consisted of epithelioid intermediate trophoblastic cells that were mononucleated and eosinophilic, or showed clear cytoplasm on microscopic examination. One case presented with a focal choriocarcinoma component. Immunohistochemically, the tumors displayed diffuse positivity for cytokeratin 18, E-cadherin, epidermal growth factor receptor, and p53 and focal positivity for p63 and hCG. However, expression of α-inhibin and placental alkaline phosphatase was almost negative. Tests for human placental lactogen and epithelial membrane antigen were also negative in all cases.
- Clinical features, characteristics of antibodies, and immunohistochemical staining results are summarized in Tables 1-3.
- Clinical history and pathologic findings
- A 41-year-old woman (gravid 2, para 2) was referred to the gynecology clinic due to vaginal bleeding. Her previous pregnancy was at term and uncomplicated. Ultrasonography demonstrated old placental remnants and dystrophic calcification. Levels of human chorionic gonadotropin (hCG) were slightly elevated (131.57 mIU/mL). The patient underwent curettage initially. Four days after the curettage, the patient visited the emergency room due to persistent abdominal pain. Uterine perforation was suspected, and total abdominal hysterectomy was performed. After the hysterectomy, her serum hCG level return to normal (1 mIU/mL).
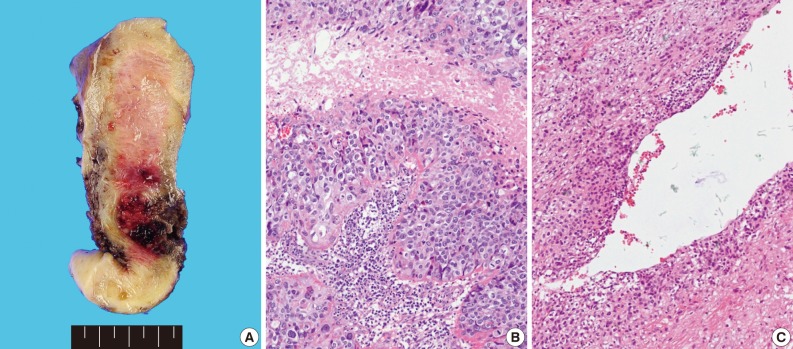
- Grossly, the tissue volume obtained by curettage was 15 mL and a remnant mass in the size of 2.5×2.0 cm was identified in the lower uterine segment of the hysterectomy specimen. The tumor was focally hemorrhagic and fragile due to necrosis (Fig. 1A). Microscopically, the tumor displayed nodular expansile growth of atypical mononucleated epithelioid trophoblastic cells lacking a dimorphic pattern (Fig. 1B). Larger cells with bizarre nuclei were occasionally observed. Necrosis and frequent mitotic activities were identified. The tumor cells surrounded the blood vessels, but did not invade the vascular lumen (Fig. 1C).
- A 32-year-old woman (gravid 3, para 1, artificial abortion 2) presented with a 3-month history of intermittent vaginal bleeding. Her previous pregnancy was 1 year prior, which was terminated by artificial abortion. Her hCG level was 313 mIU/mL. The patient underwent curettage and was diagnosed with choriocarcinoma. She was treated with several different chemotherapy regimens, due to persistent elevated serum hCG level. The main combination chemotherapy regimens included methotrexate (MTX)-leucovorin (1 cycle), actinomycin D (Act-D; 3 cycles), EMA/CO (etoposide+MTX+Act-D/cyclophosphamide and vincristine; 4 cycles), and EMA/EP (etoposide+MTX+Act-D/etoposide and cisplatin; 4 cycles), but her serum hCG level continued to be elevated to 945 mIU/mL. The patient underwent total abdominal hysterectomy with left-sided salpingoophorectomy, after which her serum hCG levels returned to normal (1.8 mIU/mL).
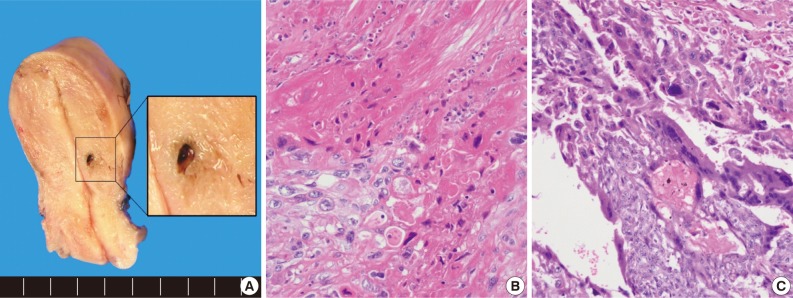
- The uterus revealed a residual small hemorrhagic mass-like lesion measuring 0.8×0.4 cm (Fig. 2A). On microscopic examination, the mass was revealed as a nodular lesion consisting of mononucleated epithelioid intermediate trophoblastic cells with hyalinization, which was reminiscent of keratinizing squamous cell carcinoma (Fig. 2B). There was also a focal choriocarcinoma component (Fig. 2C). Retrospective review of a previous curettage slide demonstrated several nests of relatively uniform intermediate trophoblastic cells.
- The third patient was referred to our consultation service from a local clinic. The patient was 42 years of age with obstetrical history of gravid 2 and para 2. The patient presented with vaginal bleeding and abdominal pain. Her last pregnancy, which was 15 years prior, had resulted in a full-term spontaneous vaginal delivery. Her serum hCG level was slightly elevated to 138.90 mIU/mL. On pelvic examination, a mass protruding from the cervical canal was discovered. The patient underwent hysterectomy and chemotherapy.
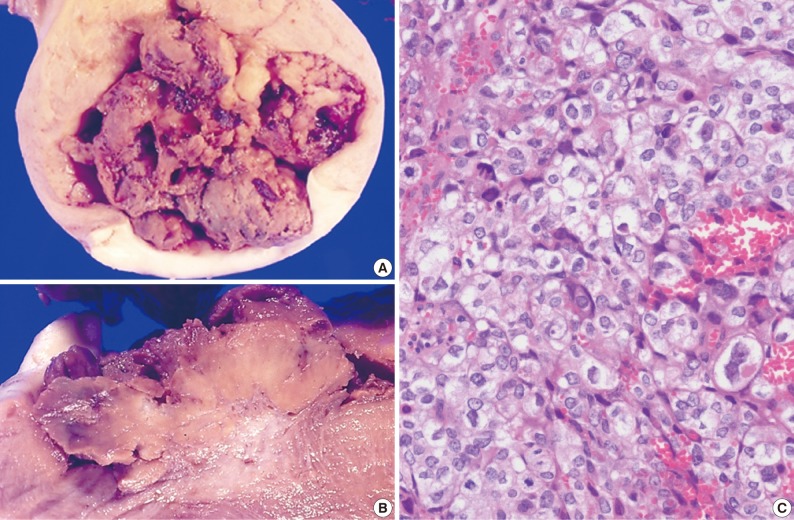
- The size of the recovered tumor was 3.8×3.3 cm (Fig. 3A, B). On microscopic examination, the tumor was composed of islands of epithelioid trophoblastic cells. The tumor cells had polygonal nuclei with moderate nuclear pleomorphism (Fig. 3C). There was no dual population of the tumor cells.
- The results of immunohistochemistry
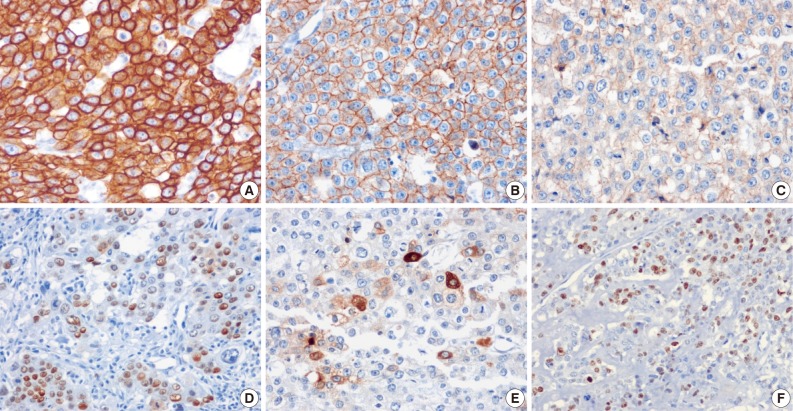
- The tumor cells presented in the three cases showed diffuse positivity for cytokeratin 18, E-cadherin, and epidermal growth factor receptor (Fig. 4A-C). The percentages of tumor cells showing p53 and p63 expression in cases 1, 2, and 3 were about 20%, 40%, and 20% and 20%, 50%, and 20%, respectively (Fig. 4D). hCG was focally positive in all cases (Fig. 4E). Inhibin showed focal positivity in tumor cells of case 2 but negativity in cases 1 and 3. Expression of human placental lactogen (hPL), placental alkaline phosphatase, and epithelial membrane antigen were negative in all cases. The MIB-1 labeling index was 36.1%, 20.9%, and 24.7%, in cases 1, 2, and 3, respectively (Fig. 4F). The syncytiotrophoblastic cells in the choriocarcinoma component of case 2 showed a more diffusely strong positive reaction in inhibin, hCG and hPL expression, in contrast to the tumor cells of the ETT component.
CASE REPORT
Case 1
Case 2
Case 3
- The clinical history of the three cases presented, summarized in Table 1, correspond to previous reported cases.1,4,7 Most of the reported cases have occurred in women of reproductive age, ranging from 15 to 48-years of age (mean, 36.1 years).7 Only one ETT case was reported to occur in a postmenopausal woman.8 The most common presenting symptom was abnormal vaginal bleeding. The patients in these cases were all of reproductive age and presented with vaginal bleeding. ETT is usually associated with a previous gestational event that includes full-term delivery (67%), spontaneous abortion (16%), and hydatidiform moles (16%).1 In accordance with these statistics, the gestational history of our cases was full-term delivery in two cases and artificial abortion in one case. The interval range between gestation and diagnosis of ETT was reported to be between 1 and 18 years (average, 6.2 years).4 The present intervals ranged from 1-15 years. Serum hCG levels were always elevated but generally low (<2,500 mIU/mL) at the time of diagnosis,7 which is consistent with our cases. The tumor mainly involves the lower uterine segment and endocervix.
- Microscopic findings in our cases were consistent with previous studies reporting ETT. The majority of ETTs show a circumscribed pushing margin and typically lack the dimorphic pattern characteristic of choriocarcinoma. ETT comprises of monomorphic chorionic-type intermediate trophoblastic cells,7 which resemble intermediate trophoblastic cells of chorion laeve of the normal placenta both histologically and immunohistochemically.7,9 The present immunohistochemical findings were entirely consistent with these features. The tumor cells were relatively uniform in size and mononucleated with round nuclei and eosinophilic or clear cytoplasm and grew in cords, sheets, and nests. A distinct hyaline-like material and necrosis could be identified.
- The differential diagnoses of ETT include choriocarcinoma, PSTT, PSN, and keratinizing squamous cell carcinoma of the cervix. Choriocarcinoma displays a dimorphic population of trophoblast cells (syncytiotroblastic cells and cytotrophoblastic or intermediate trophoblastic cells) with marked hemorrhage and necrosis that is readily observed grossly. ETT is usually not associated with marked hemorrhage. The nodular growth pattern of ETT contrasts with the diffuse infiltrative growth pattern of PSTT. ETT cells are smaller than the intermediate trophoblast cells of PSTT. Immunohistochemistry is helpful in distinguishing between these two entities. The trophoblastic cells of PSTT are strongly immunoreactive for hPL and Mel-CAM, whereas the majority of ETT are negative for these markers.10 The chorionic type trophoblasts that comprise ETT are positive for p63 in many cases (45-80%), but the implantation type of trophoblasts in PSTT are not.10,11 Expression of p63 in ETT is variable.10 In the present report, two cases expressed for p63 in less than 25% of cell, while one case showed positivity in about 50%. PSN presents a microscopic-sized, well-circumscribed nodule with low cellularity, while ETT is larger, more cellular and necrotic. Despite the distinct morphologic features and immunoprofiles that have been described for each of these ITTs, ITT shows overlapping morphologic features and immunoprofiles, as well as sharing similar clinical behavior, treatment, and outcome. Therefore, some say that it is inconsequential to distinguish between ETT and PSTT.10
- ETT can be misdiagnosed as squamous cell carcinoma of the cervix because of their epithelioid appearance and the resemblance of the hyaline and necrotic debris to keratin, especially when tumors are located in the cervix and the lower uterine segment. We have previously misinterpreted ETT as cervical squamous cell carcinoma in Pap smear cytology.12 Immunohistochemistry for inhibin and cytokeratin 18 can be useful, as these markers are positive in ETT but negative in squamous cell carcinoma.7,9 However, the percentage and intensity of positive cells for inhibin in ITTs are limited. Only about 40% of ITT display focal immunoreactivity for inhibin and this reaction can be completely absent in some cases.10 Cases 1 and 3 were negative for inhibin, while case 2 showed focally positive areas for inhibin.
- In addition, the Ki-67 proliferative index helps in differential diagnosis, as it is very high (>50%) in choriocarcinoma and squamous cell carcinoma and relatively lower in PSTT (15-25%), ETT (10-25%), and PSN (<10%).13 However, several ETT cases showing a high mitotic index and Ki-67 labeling index have been reported.2,8 A high mitotic index has been suspected to be associated with the malignant behavior of ETT.8,14 Case 1 showed active mitotic activity (36/10 high power filed) and MIB-1 labeling index (36.1%). This patient has had no recurrence or metastasis during 8 years of follow-up.
- Mixed trophoblastic tumors with morphologic and immunohistochemical features of ETT, PSTT, and choriocarcinoma in the same tumor have been described in several studies.6,7,10,14 Case 2 had a focal area of choriocarcinoma consisting of multinucleated syncytiotrophoblastic cells and revealed different immunohistochemical findings in several markers compared with ETT cells. In contract to a previous study, inhibin and hPL showed stronger and more diffuse positive reactions in the choriocarcinoma area.10
- ETT has been reported to be similar to PSTT in terms of prognosis and behavior. Metastasis and death have been reported in 25% and 10% of patients with ETT, respectively.7 Pathologic features such as tumor size, percentage of tumor necrosis, and cytological atypia may not be related to the prognosis. However, mitotic activity is suspected to be associated with malignant behavior of ETT.8,13 Nagai et al.15 reported the usefulness of p53 immunostaining in predicting of invasive or recurrent propensity of PSTT cells. Two ETT cases were positive for p53, which is involved in the biology of ETT cells. The three cases presented here also showed positivity for p53. However, further investigation is warranted to establish a relationship between p53 expression and prognosis. ETT does not respond to chemotherapeutic agents used for other types of gestational trophoblastic tumor,1,16,17 and surgical resection is recommended as the primary treatment.1
- In summary, we have described three cases of unusual trophoblastic tumors. Since the prognosis and treatment of ETT are different from those of squamous cell carcinoma and choriocarcinoma, accurate diagnosis is essential. Detailed light microscopic examination and immunochemical study are helpful in establishing an accurate diagnosis in a timely manner.
DISCUSSION
- 1. Shih IM, Mazur MT, Kurman RJ. In: Kurman RJ, ed. Gestational trophoblastic disease and related lesions. Blaustein's pathology of the female genital tract. 2002; 5th ed. New York: Springer, 1193-1247.
- 2. Shih IM, Kurman RJ. The pathology of intermediate trophoblastic tumors and tumor-like lesions. Int J Gynecol Pathol 2001; 20: 31-47. ArticlePubMed
- 3. Mazur MT, Lurain JR, Brewer JI. Fatal gestational choriocarcinoma: clinicopathologic study of patients treated at a trophoblastic disease center. Cancer 1982; 50: 1833-1846. ArticlePubMed
- 4. Mazur MT. Metastatic gestational choriocarcinoma: unusual pathologic variant following therapy. Cancer 1989; 63: 1370-1377. PubMed
- 5. Jones WB, Romain K, Erlandson RA, Burt ME, Lewis JL Jr. Thoracotomy in the management of gestational choriocarcinoma: a clinicopathologic study. Cancer 1993; 72: 2175-2181. PubMed
- 6. Silva EG, Tornos C, Lage J, Ordonez NG, Morris M, Kavanagh J. Multiple nodules of intermediate trophoblast following hydatidiform moles. Int J Gynecol Pathol 1993; 12: 324-332. PubMed
- 7. Shih IM, Kurman RJ. Epithelioid trophoblastic tumor: a neoplasm distinct from choriocarcinoma and placental site trophoblastic tumor simulating carcinoma. Am J Surg Pathol 1998; 22: 1393-1403. PubMed
- 8. Coulson LE, Kong CS, Zaloudek C. Epithelioid trophoblastic tumor of the uterus in a postmenopausal woman: a case report and review of the literature. Am J Surg Pathol 2000; 24: 1558-1562. PubMed
- 9. Shih IM, Seidman JD, Kurman RJ. Placental site nodule and characterization of distinctive types of intermediate trophoblast. Hum Pathol 1999; 30: 687-694. PubMed
- 10. Kalhor N, Ramirez PT, Deavers MT, Malpica A, Silva EG. Immunohistochemical studies of trophoblastic tumors. Am J Surg Pathol 2009; 33: 633-638. PubMed
- 11. Shih IM, Kurman RJ. p63 expression is useful in the distinction of epithelioid trophoblastic and placental site trophoblastic tumors by profiling trophoblastic subpopulations. Am J Surg Pathol 2004; 28: 1177-1183. PubMed
- 12. Lee EJ, Lee HW, Lee JS, et al. A case of epithelioid trophoblastic tumor. Korean J Gynecol Oncol Colposc 2001; 12: 152-155.
- 13. Shih IM, Kurman RJ. Ki-67 labeling index in the differential diagnosis of exaggerated placental site, placental site trophoblastic tumor, and choriocarcinoma: a double immunohistochemical staining technique using Ki-67 and Mel-CAM antibodies. Hum Pathol 1998; 29: 27-33. PubMed
- 14. Fadare O, Parkash V, Carcangiu ML, Hui P. Epithelioid trophoblastic tumor: clinicopathological features with an emphasis on uterine cervical involvement. Mod Pathol 2006; 19: 75-82. PubMed
- 15. Nagai Y, Kamoi S, Matsuoka T, et al. Impact of p53 immunostaining in predicting advanced or recurrent placental site trophoblastic tumors: a study of 12 cases. Gynecol Oncol 2007; 106: 446-452. PubMed
- 16. Twiggs LB, Hartenbach E, Saltzman AK, King LA. Metastatic placental site trophoblastic tumor. Int J Gynaecol Obstet 1998; 60(Suppl 1):S51-S55. PubMed
- 17. Knox S, Brooks SE, Wong-You-Cheong J, Ioffe O, Meisenberg B, Goldstein DP. Choriocarcinoma and epithelial trophoblastic tumor: successful treatment of relapse with hysterectomy and high-dose chemotherapy with peripheral stem cell support: a case report. Gynecol Oncol 2002; 85: 204-208. PubMed
REFERENCES
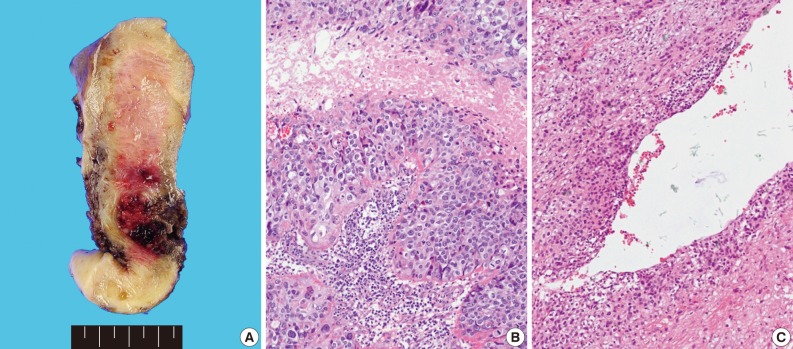
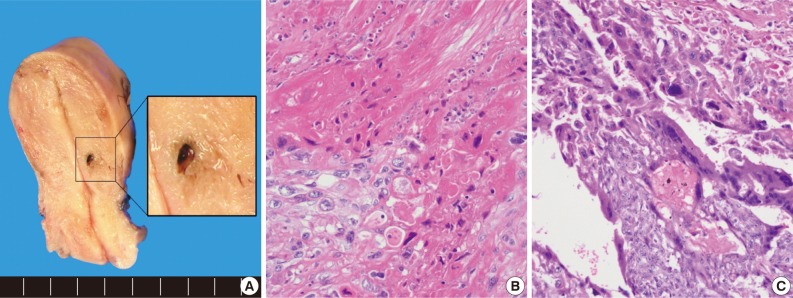
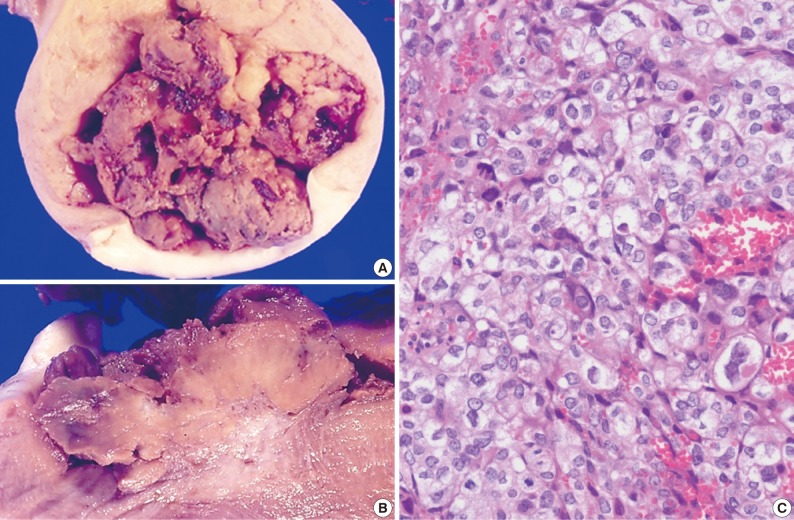
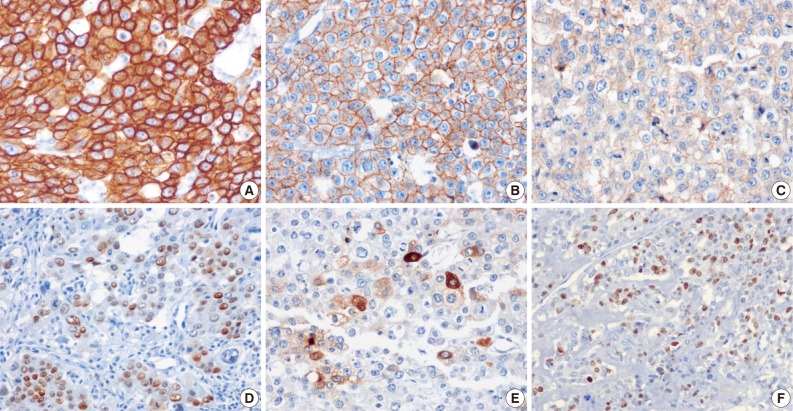
Figure & Data
References
Citations

- Epithelioid Trophoblastic Tumour: A Case with Genetic Linkage to a Child Born over Seventeen Years Prior, Successfully Treated with Surgery and Pembrolizumab
David Pisani, Jean Calleja-Agius, Riccardo Di Fiore, John J. O’Leary, James P. Beirne, Sharon A. O’Toole, Ana Felix, Ian Said-Huntingford
Current Oncology.2021; 28(6): 5346. CrossRef - Epithelioid Trophoblastic Tumor
Stephanie M. McGregor, Larissa V. Furtado, Anthony G. Montag, Rebecca Brooks, Ricardo R. Lastra
International Journal of Gynecological Pathology.2020; 39(1): 8. CrossRef - Epithelioid trophoblastic tumor in a postmenopausal woman: A case report and review of the literature in the postmenopausal group
Seyran Yigit, Eylul Gun, Bulent Yilmaz, Zafer Kolsuz
Indian Journal of Pathology and Microbiology.2020; 63(5): 98. CrossRef - Double trouble: Extrauterine epithelioid trophoblastic tumor with uterine choriocarcinoma - An autopsy report
Kusum Jashnani, Alshifa Yagana, Niraj Mahajan
Indian Journal of Cancer.2020;[Epub] CrossRef - Epithelioid trophoblastic tumor coexisting with choriocarcinoma around an abdominal wall cesarean scar: a case report and review of the literature
Chunfeng Yang, Jianqi Li, Yuanyuan Zhang, Hanzhen Xiong, Xiujie Sheng
Journal of Medical Case Reports.2020;[Epub] CrossRef - Placental site trophoblastic tumor and epithelioid trophoblastic tumor: Clinical and pathological features, prognostic variables and treatment strategy
Angiolo Gadducci, Silvestro Carinelli, Maria Elena Guerrieri, Giovanni Damiano Aletti
Gynecologic Oncology.2019; 153(3): 684. CrossRef - Diagnosis and Management of Mixed Gestational Trophoblastic Neoplasia: A Study of 16 Cases and a Review of the Literature
Yujia Kong, Guangshi Tao, Liju Zong, Junjun Yang, Xirun Wan, Wenze Wang, Yang Xiang
Frontiers in Oncology.2019;[Epub] CrossRef - A Case Series of Five Patients With Pure or Mixed Gestational Epithelioid Trophoblastic Tumors and a Literature Review on Mixed Tumors
Ka Yu Tse, Keith Wan Hang Chiu, Karen Kar Loen Chan, Mandy Man Yee Chu, Siew Fei Ngu, Annie Nga Yin Cheung, Hextan Yuen Sheung Ngan, Philip Pun Ching Ip
American Journal of Clinical Pathology.2018; 150(4): 318. CrossRef - MR Imaging of Uterine Epithelioid Trophoblastic Tumor: A Case Report
Sakiko KAGEYAMA, Masafumi KANOTO, Yukio SUGAI, Takeshi SUTO, Satoru NAGASE, Mitsumasa OSAKABE, Takaaki HOSOYA
Magnetic Resonance in Medical Sciences.2016; 15(4): 411. CrossRef - Pharmacotherapy of placental site and epithelioid trophoblastic tumours
Fiona Taylor, Barry W Hancock
Expert Opinion on Orphan Drugs.2015; 3(1): 75. CrossRef - A Well-Circumscribed Border with Peripheral Doppler Signal in Sonographic Image Distinguishes Epithelioid Trophoblastic Tumor from Other Gestational Trophoblastic Neoplasms
Jiale Qin, Weiwen Ying, Xiaodong Cheng, Xiaodong Wu, Bingjian Lu, Yun Liang, Xinyu Wang, Xiaoyun Wan, Xing Xie, Weiguo Lu, Hai-Yan Lin
PLoS ONE.2014; 9(11): e112618. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
- Related articles




Fig. 1
Fig. 2
Fig. 3
Fig. 4
| Case No. | Age (yr) | Symptom | Recent gestation history | Tumor size (cm) | Serum hCG (mIU/mL) | Treatment | FIGO stage | Follow-up (yr) | Metastasis | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 41 | Vaginal bleeding | FTD, unknown | 2.5 × 2.0, 15 mL (C) | 131.57 | TAH | I | 8 | No | CR |
| 2 | 32 | Vaginal bleeding | AA, 1 yr ago | 0.8 × 0.4, 25 mL (C) | 313.00 | CTx, TAH | I | 4 | No | CR |
| 3 | 42 | Vaginal bleeding | FTD, 15 yr ago | 3.8 × 3.3 | 138.90 | TAH, CTx | I | No data | No (initial) | No data |
| Antibody | Source | Dilution | Antigen retrieval | Primary antibody incubation time |
|---|---|---|---|---|
| CK18 | Dako | 1 : 200 | Mild (99°C, 30 min) | 24 min |
| p63 | Dako | 1 : 150 | Mild (99°C, 30 min) | 16 min |
| Inhibin | Dako | 1 : 40 | Standard (99°C, 60 min) | 52 min |
| PLAP | Zymed | 1 : 50 | Standard (99°C, 60 min) | 1 hr 32 min |
| hPL | Dako | Prediluted | Standard (99°C, 60 min) | 1 hr |
| hCG | Biomedia | Prediluted | Standard (99°C, 60 min) | 24 min |
| EGFR | Ventana | Prediluted | Protease I (8 min) | 20 min |
| E-cadherin | Zymed | 1 : 100 | Standard (99°C, 60 min) | 1 hr |
| p53 | Ventana | Prediluted | Mild (99°C, 30 min) | 20 min |
| EMA | Dako | 1 : 60 | Standard (99°C, 60 min) | 1 hr |
| MIB-1 | Dako | 1 : 70 | Standard (99°C, 60 min) | 1 hr 20 min |
| Case No. | CK18 | p63 | Inhibin | PLAP | hPL | hCG | EGFR | E-cad | p53 | EMA | MIB-1 LI (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | + | +, f | - | - | - | + | + | + | + | - | 36.1 |
| 2 | + | + | +, f | - | - | +, f | + | + | + | - | 20.9 |
| 3 | + | +, f | - | - | - | + | + | + | + | - | 24.7 |
hCG, human chorionic gonadotropin; FIGO, The International Federation of Gynecology and Obstetrics; FTD, full term delivery; C; curettage; TAH, total abdominal hysterectomy; CR, complete remission; AA, artificial abortion; CTx, chemotherapy.
CK, cytokeratin; PLAP, placental alkaline phosphatase; hPL, human placental lactogen; hCG, human chorionic gonadotropin; EGFR, epidermal growth factor receptor; EMA, epithelial membrane antigen.
CK, cytokeratin; PLAP, placental alkaline phosphatase; hPL, human placental lactogen; hCG, human chorionic gonadotropin; EGFR, epidermal growth factor receptor; E-cad, E-cadherin; EMA, epithelial membrane antigen; LI, labeling index; f, focal.

 E-submission
E-submission