Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 47(3); 2013 > Article
-
Original Article
Proposal for a Standardized Pathology Report of Gastroenteropancreatic Neuroendocrine Tumors: Prognostic Significance of Pathological Parameters - , Mee-Yon Cho1, Jin Hee Sohn2, So Young Jin3, Hyunki Kim4, Eun Sun Jung5, Mi-Jung Kim6,, Kyoung-Mee Kim7, Woo Ho Kim8, Joon Mee Kim9, Yun Kyung Kang10, Joon Hyuk Choi11, Dae Young Kang12, Youn Wha Kim13, Eun Hee Choi14The Gastrointestinal Pathology Study Group of Korean Society of Pathologists
-
Korean Journal of Pathology 2013;47(3):227-237.
DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.3.227
Published online: June 25, 2013
1Department of Pathology, Wonju Severance Christian Hospital, Yonsei University Wonju College of Medicirne, Wonju, Korea.
2Department of Pathology, Kangbuk Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
3Department of Pathology, Soon Chun Hyang University Hospital, Soon Chun Hyang University College of Medicine, Seoul, Korea.
4Department of Pathology, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
5Department of Pathology, Seoul St. Mary's Hospital, The Catholic University of Korea, College of Medicine, Seoul, Korea.
6Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
7Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
8Department of Pathology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
9Department of Pathology, Inha University Hospital, Inha University School of Medicine, Incheon, Korea.
10Department of Pathology, Inje University Seoul Paik Hospital, Inje University College of Medicine, Seoul, Korea.
11Department of Pathology, Yeungnam University Medical Center, Yeungnam University College of Medicine, Daegu, Korea.
12Department of Pathology, Chungnam National University Hospital, Chungnam National University School of Medicine, Daejeon, Korea.
13Department of Pathology, Kyunghee University Medical Center, Kyung Hee University School of Medicine, Seoul, Korea.
14Division of Statistics in Institute of Lifestyle Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea.
- Corresponding Author: Jin Hee Sohn, M.D. Department of Pathology, Kangbuk Samsung Hospital, Sungkyungkwan University School of Medicine, 29 Saemunan-ro, Jongno-gu, Seoul 110-746, Korea. Tel: +82-2-2001-2391, Fax: +82-2-2001-2398, jhpath.sohn@samsung.com
- *Mi-Jung Kim's present address: Department of Pathology, Daehang Hospital, Seoul, Korea.
© 2013 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations

- Analysis of Prognostic Risk Factors of Endoscopic Submucosal Dissection (ESD) and Curative Resection of Gastrointestinal Neuroendocrine Neoplasms
Yuan Si, ChaoKang Huang, JingBin Yuan, XianHui Zhang, QingQiang He, ZhiJin Lin, Ling He, ZhongXin Liu, Yuvaraja Teekaraman
Contrast Media & Molecular Imaging.2022;[Epub] CrossRef - Standardization of the pathologic diagnosis of appendiceal mucinous neoplasms
Dong-Wook Kang, Baek-hui Kim, Joon Mee Kim, Jihun Kim, Hee Jin Chang, Mee Soo Chang, Jin-Hee Sohn, Mee-Yon Cho, So-Young Jin, Hee Kyung Chang, Hye Seung Han, Jung Yeon Kim, Hee Sung Kim, Do Youn Park, Ha Young Park, So Jeong Lee, Wonae Lee, Hye Seung Lee,
Journal of Pathology and Translational Medicine.2021; 55(4): 247. CrossRef - Preoperative diagnosis of well‐differentiated neuroendocrine tumor in common hepatic duct by brush cytology: A case report
Jiwoon Choi, Kyong Joo Lee, Sung Hoon Kim, Mee‐Yon Cho
Diagnostic Cytopathology.2019; 47(7): 720. CrossRef - Primary renal well-differentiated neuroendocrine tumors: report of six cases with an emphasis on the Ki-67 index and mitosis
Bohyun Kim, Han-Seong Kim, Kyung Chul Moon
Diagnostic Pathology.2019;[Epub] CrossRef - Primary low‐grade neuroendocrine carcinoma of the skin: An exceedingly rare entity
Tiffany Y. Chen, Annie O. Morrison, Joe Susa, Clay J. Cockerell
Journal of Cutaneous Pathology.2017; 44(11): 978. CrossRef - Prognostic Validity of the American Joint Committee on Cancer and the European Neuroendocrine Tumors Staging Classifications for Pancreatic Neuroendocrine Tumors
Jae Hee Cho, Ji Kon Ryu, Si Young Song, Jin-Hyeok Hwang, Dong Ki Lee, Sang Myung Woo, Young-Eun Joo, Seok Jeong, Seung-Ok Lee, Byung Kyu Park, Young Koog Cheon, Jimin Han, Tae Nyeun Kim, Jun Kyu Lee, Sung-Hoon Moon, Hyunjin Kim, Eun Taek Park, Jae Chul Hw
Pancreas.2016; 45(7): 941. CrossRef - Early diagnosis and treatment of gastrointestinal neuroendocrine tumors
Hong Shen, Zhuo Yu, Jing Zhao, Xiu-Zhen Li, Wen-Sheng Pan
Oncology Letters.2016; 12(5): 3385. CrossRef - Recent Updates on Neuroendocrine Tumors From the Gastrointestinal and Pancreatobiliary Tracts
Joo Young Kim, Seung-Mo Hong
Archives of Pathology & Laboratory Medicine.2016; 140(5): 437. CrossRef - Pancreatic neuroendocrine tumors: Correlation between the contrast-enhanced computed tomography features and the pathological tumor grade
Koji Takumi, Yoshihiko Fukukura, Michiyo Higashi, Junnichi Ideue, Tomokazu Umanodan, Hiroto Hakamada, Ichiro Kanetsuki, Takashi Yoshiura
European Journal of Radiology.2015; 84(8): 1436. CrossRef - Tumeurs neuroendocrines du tube digestif et du pancréas : ce que le pathologiste doit savoir et doit faire en 2014
Jean-Yves Scoazec, Anne Couvelard
Annales de Pathologie.2014; 34(1): 40. CrossRef - Spectrum of Gastroenteropancreatic NENs in Routine Histological Examinations of Bioptic and Surgical Specimen: A Study of 161 Cases Collected from 17 Departments of Pathology in the Czech Republic
Václav Mandys, Tomáš Jirásek
Gastroenterology Research and Practice.2014; 2014: 1. CrossRef - p27 Loss Is Associated with Poor Prognosis in Gastroenteropancreatic Neuroendocrine Tumors
Hee Sung Kim, Hye Seung Lee, Kyung Han Nam, Jiwoon Choi, Woo Ho Kim
Cancer Research and Treatment.2014; 46(4): 383. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
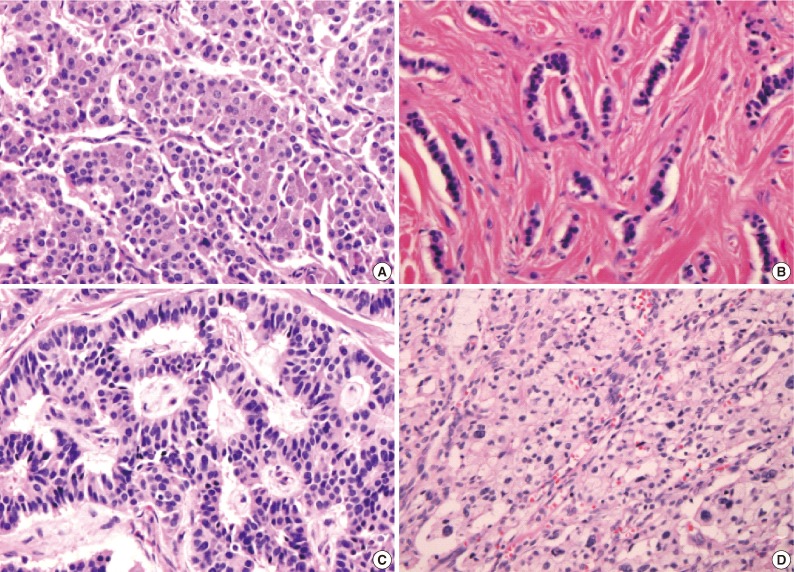
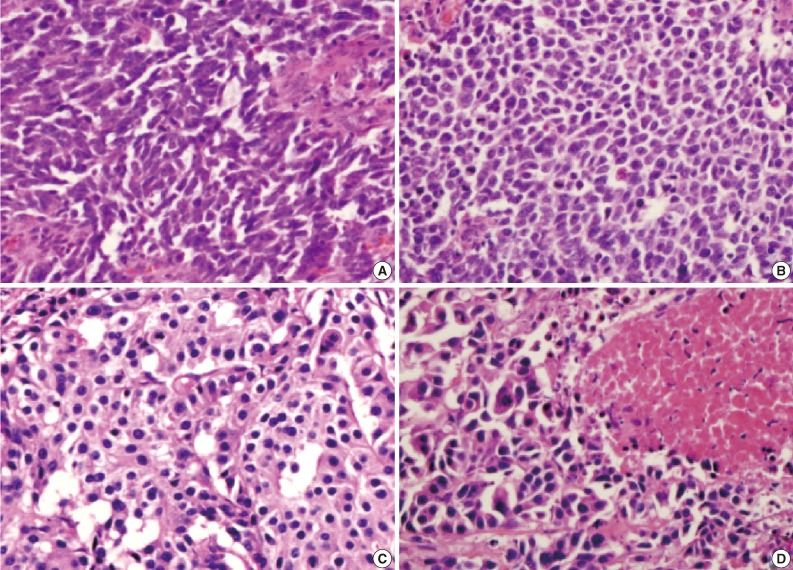
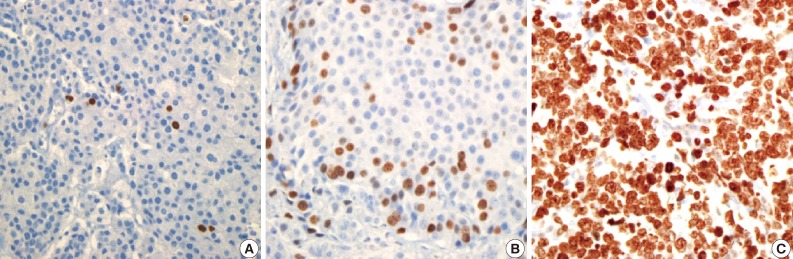
- Figure










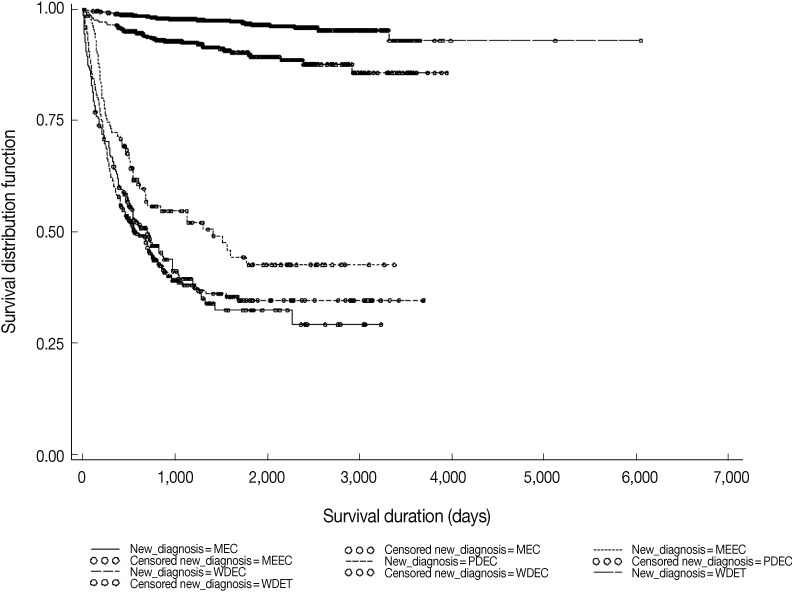
Fig. 1
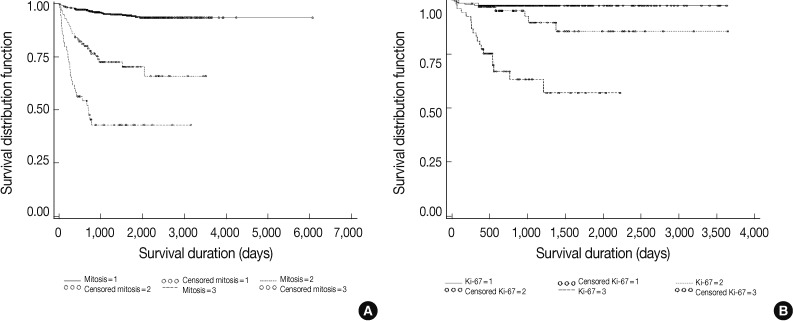
Fig. 2
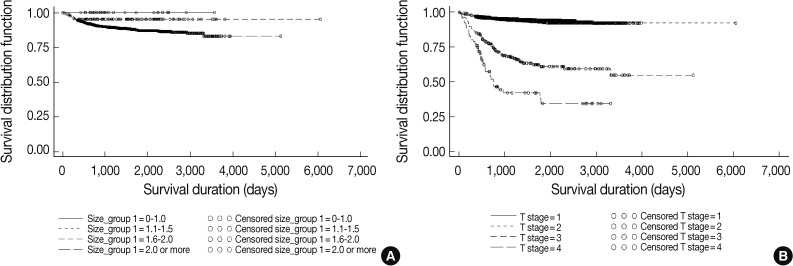
Fig. 3
Fig. 4
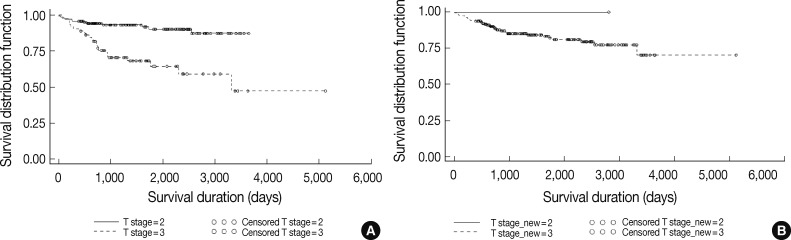
Fig. 5
Fig. 6
Fig. 7
Fig. 8
Fig. 9
Fig. 10
| Parameter | No. of cases recorded |
|---|---|
| Mean age (yr) | 54.66 |
| Sex (male:female) | 1.5:1 |
| Diagnosis with site | 3,631 |
| Tumor size | 3,045 |
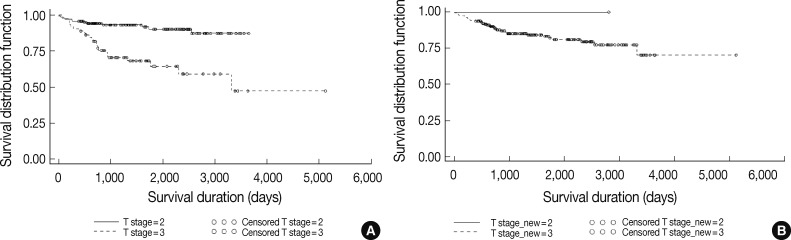
| Extent | 3,440 |
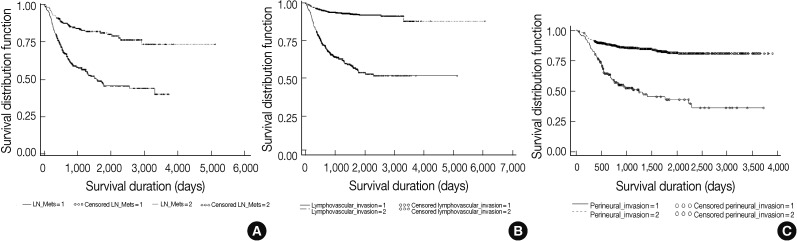
| Lymph node metastasis | 791 |
| Distant metastasis | 2,094 |
| Lymphovascular invasion | 2,312 |
| Perineural invasion | 1,128 |
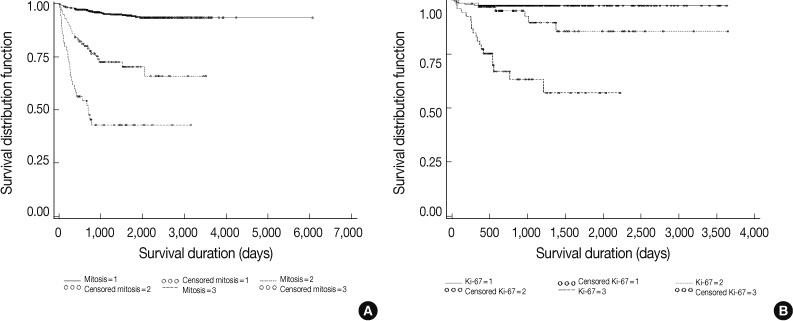
| Grading by mitosis | 1,598 |
| Grading by Ki-67 labeling index | 637 |
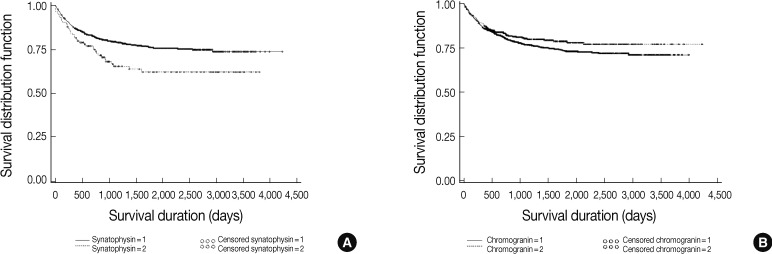
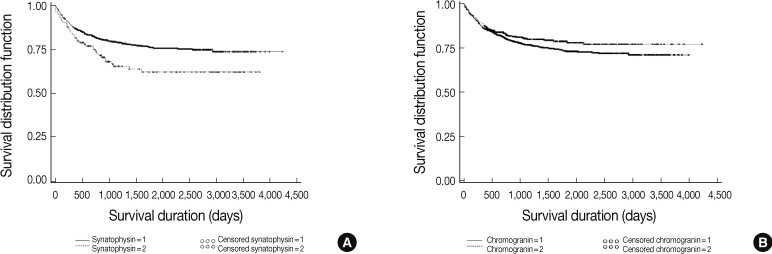
| Immunohistochemical stain: synaptophysin | 2,410 |
| Immunohistochemical stain: chromogranin | 2,455 |
| Survival | 3,312 |
| Size | Mitosis | Ki-67 labeling index | |
|---|---|---|---|
| Size | 1.00000 | - | - |
| - | - | - | |
| 3,050 | - | - | |
| Mitosis | 0.43269 | 1.00000 | - |
| < 0.0001 | - | - | |
| 1,301 | 1,594 | - | |
| Ki-67 labeling index | 0.37809 | 0.66960 | 1.00000 |
| < 0.0001 | < 0.0001 | - | |
| 526 | 336 | 636 |
| T category | Grade of mitosis (%) |
|||
|---|---|---|---|---|
| 1 | 2 | 3 | Total | |
| 1 | 245 (18.79) | 3 (0.97) | 1 (2.04) | 249 (17.13) |
| 2 | 1,021 (78.30) | 61 (60.40) | 20 (40.82) | 1,102 (75.79) |
| 3 | 37 (2.84) | 29 (2.71) | 16 (32.65) | 82 (5.64) |
| 4 | 1 (0.08) | 8 (7.92) | 12 (24.49) | 21 (1.44) |
| Total | 1,304 (100) | 101 (100) | 49 (100) | 100 (100) |
| AJCC | ENETS |
||||
|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | Total | |
| T1 | 1,262 | 0 | 0 | 0 | 1,262 |
| T2 | 0 | 2,073 | 295 | 0 | 2,368 |
| T3 | 0 | 0 | 287 | 0 | 287 |
| T4 | 0 | 0 | 0 | 84 | 84 |
| Total | 1,262 | 2,073 | 582 | 84 | 4,001 |
| AJCC | ENETS |
||
|---|---|---|---|
| T2 | T3 | Total | |
| T2 | 6 | 295 | 301 |
| T3 | 0 | 88 | 88 |
| Total | 6 | 363 | 389 |
| Definition | ENETS | AJCC |
|---|---|---|
| T1 | Limited to the pancreas, < 2 cm | Limited to the pancreas, ≤ 2 cm |
| T2 | Limited to the pancreas, 2-4 cm | Limited to the pancreas, > 2 cm |
| T3 | Limited to the pancreas > 4 cm, or invading duodenum or bile duct | Beyond the pancreas, but without involvement of the superior mesenteric artery |
| T4 | Peripancreatic spread with invasion of large vessels or adjacent organs | Vascular invasion (celiac axis or the superior mesenteric artey) |
Simple kappa value, 0.8735; weighted kappa value, 0.8949.
Simple kappa value, 0.0091.

 E-submission
E-submission