Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 47(3); 2013 > Article
-
Original Article
Aspiration Cytopathology of Peripancreatic Space: A Clinicoradiologic and Cytopathologic Analyses of 42 Cases - Justin Bishop, Wei Zhang1, Olga B. Ioffe1, Syed Z. Ali
-
Korean Journal of Pathology 2013;47(3):258-264.
DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.3.258
Published online: June 25, 2013
Department of Pathology, The Johns Hopkins Hospital, Baltimore, MD, USA.
Department of Pathology, University of Maryland Medical Center, Baltimore, MD, USA.
- Corresponding Author: Syed Z. Ali, M.D. Department of Pathology, The Johns Hopkins Hospital, 600 North Wolfe Street, Path 406, Baltimore, MD 21287, USA. Tel: +1-410-955-1180, Fax: +1-410-614-9556, sali@jhmi.edu
© 2013 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 9,469 Views
- 41 Download
Abstract
-
Background
- The pancreas is surrounded by soft tissue known as the peripancreatic space (PPS). Pathologic lesions of the PPS are infrequent and have only rarely been reported in the cytopathology literature.
-
Methods
- A retrospective review of cytopathology files at two large institutions revealed 42 cases of PPS lesions obtained by transabdominal fine needle aspiration (FNA) or endoscopic ultrasound-guided FNA over a 16-year period. Clinicoradiologic findings and follow-up information were also reviewed.
-
Results
- Patients ranged in age from 23-83 years (mean, 60 years) with an equal gender distribution. The major clinical presentations included pain, jaundice, nausea/vomiting, and abnormal liver enzymes. Radiographic characteristics included lymphadenopathy and cystic/solid soft tissue masses with a size range of 1.5 to 8 cm. Cytologically, 4 (9.5%) cases were nondiagnostic, 9 (21.5%) were diagnosed as benign, 4 (9.5%) were atypical or suspicious for cancer, and 25 (59.5%) were malignant. Six of 25 (24%) patients had metastasis of a prior known malignancy.
-
Conclusions
- FNA of PPS masses is a rare occurrence. The majority of lesions are metastatic carcinomas from a variety of primary sites. Flow cytometry and immunoperoxidase studies are useful adjuncts to determine the tumor origin. The sensitivity of PPS aspiration for a malignant diagnosis is 90% with a positive predictive value of 100%.
- A retrospective search of the cytopathology archives at The Johns Hopkins Hospital and University of Maryland Medical Center was carried out for a period of 16 years (1989 to 2005). The search revealed 42 FNAs obtained specifically from the peripancreatic space. Clinicoradiologic findings and follow-up information for each case were additionally reviewed and correlated with the morphologic findings.
- In 33 cases the material was obtained transabdominally via ultrasound or computed tomograhphic guidance employing a 22-gauge fine needle. In 9 cases FNA was performed through a curvilinear echoendoscope with a 22-gauge needle. All cases had on-site evaluation performed by the cytopathology staff. A standard smear technique with air-drying and staining with Diff Quik was employed. Slides were also wet-fixed in 95% ethanol for subsequent Papanicolaou staining. Needle rinsed with balanced salt solution were subsequently made into paraffin cell blocks and 4 µm sections were stained with hematoxylin and eosin. Immunoperoxidase staining was performed in 10 cases for neuroendocrine and/or lymphoid markers employing the conventional methodology. In 7 cases suspicious for a lymphoproliferative process the aspirate was also sent for flow cytometric analysis.
MATERIALS AND METHODS
- Patients' demographics, clinical, and radiologic data (Table 1)
- A total of 42 patients with a male to female ratio of 1:1 was included in the study. The ages of the patients ranged from 23-83 years (mean, 60 years; median, 64 years). Five cases were consults from outside institutions without detailed clinical or radiologic information. Of the remaining 37 cases, the major clinical presentations included upper gastrointestinal symptoms, jaundice, abdominal pain, and abnormal liver enzymes. Eleven patients (6 females and 5 males) had a prior history of malignancy including lymphoma and carcinomas of the lung, colon, breast, and kidney.
- Radiologically, 2 patients were found to have cystic lesions in the peripancreatic region. Eight patients had only peripancreatic lymphadenopathy, 17 patients had a mass or other abnormal radiologic appearance involving the pancreas and/or the peripancreatic space. Ten patients had both pancreatic involvement and diffuse peripancreatic lymphadenopathy. The sizes of the lesions ranged from 1.5 to 8.0 cm. The radiologic impressions ranged from benign lesions such as pseudocyst to metastatic malignancy.
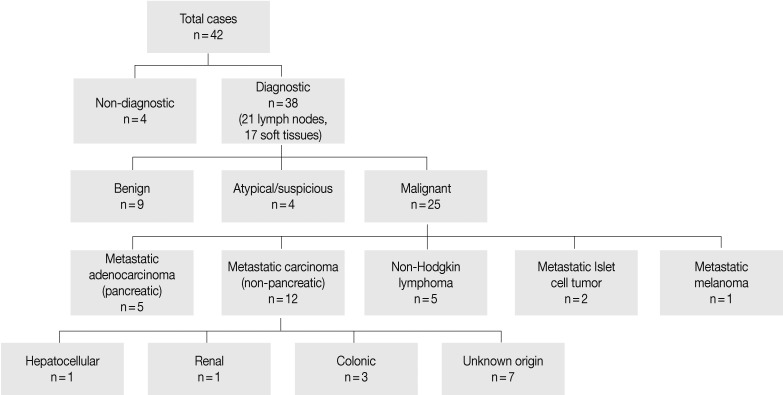
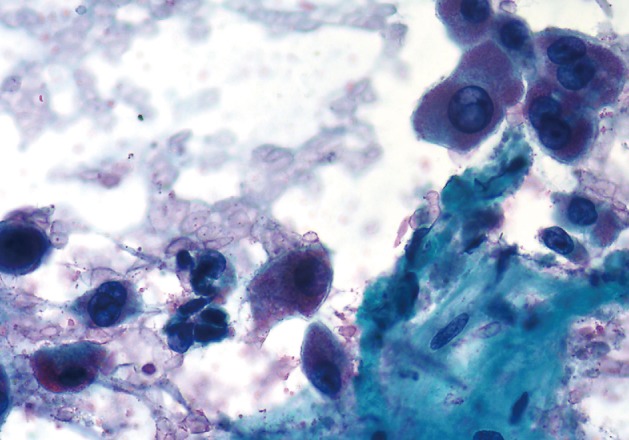
- Cytopathologic findings (Fig. 1)
- Cytologic materials were obtained as mentioned above by either EUS-FNA or CT/ultrasound guided transabdominal FNA. The sites of aspiration included the peripancreatic lymph nodes (n=12), peripancreatic soft tissue (n=18, mass or cyst), and from both lymph nodes and soft tissue (n=12).
- Four cases did not obtain adequate diagnostic materials. In later surgical resection specimens 2 of them had moderately differentiated adenocarcinoma and the other 2 had only pancreatic intraepithelial neoplasias. Of the remaining 38 cases, 9 cases were diagnosed as negative for malignancy on cytology. Three of these turned out to be malignant (2 lymphomas and 1 carcinoma) on surgical pathology follow-up. The 3 cytologically negative cases that turned up positive on follow-up had sampling issues including a small tumor size that was not sampled by the FNA needle, a common problem at any anatomic site. None of these three cases were missed on cytology. Four cases were called atypical/suspicious for malignancy on cytology and two of them proved to be carcinoma on subsequent resection or biopsy.
- The cytologic diagnoses of 25 cases called malignant on FNA included 5 metastatic adenocarcinomas of the pancreas, 5 non-Hodgkin lymphomas, 2 metastatic pancreatic endocrine (islet cell) tumors, 1 metastatic melanoma, and 12 metastatic carcinomas of a non-pancreatic origin (1 hepatocellular, 3 colon, 1 renal cell, and 7 carcinomas of unknown primary). Six of these patients had metastasis of a prior known malignancy (colon, kidney, liver, and skin). Flow cytometry was used to confirm the lymphoma cases. Five of the 10 metastatic cases were successfully analyzed by immunoperoxidase staining to identify the site of the primary tumors.
- Cytomorphologic features
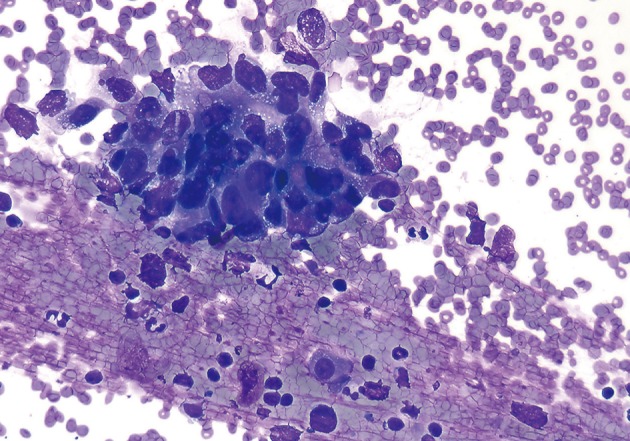
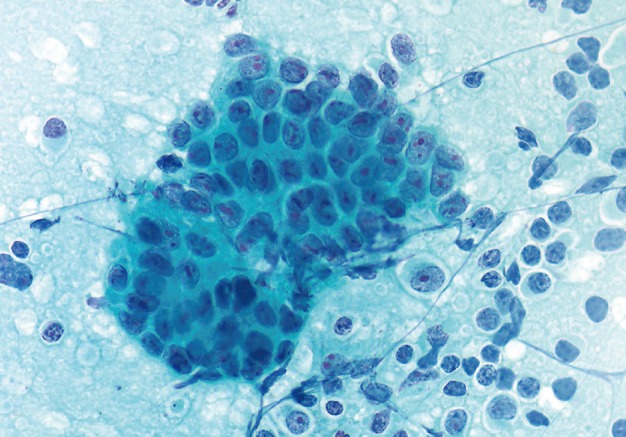
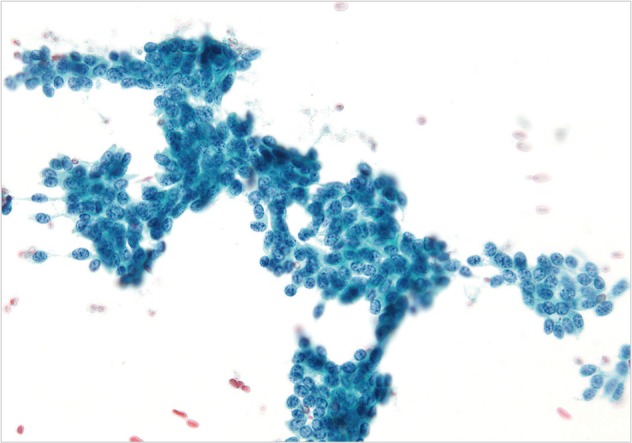
- The smears of metastatic adenocarcinoma (Figs. 2, 3) generally showed tissue fragments of varying sizes admixed with single cells, often with an associated background tumor diathesis and/or lymphocytes. The fragments were three-dimensional with a frequent glandular architecture. The malignant cells were often quite pleomorphic and displayed loss of polarity with a haphazard architecture. The cells had round to oval nuclei and occasionally prominent nucleoli.
- The cases of metastatic well-differentiated endocrine neoplasm (islet cell tumor) showed loosely cohesive fragments of monotonous cells with small round to oval uniform nuclei and speckled ("checker board") chromatin (Fig. 4). The cells displayed a rather flat monolayered architecture with lack of glandular differentiation. The one case of metastatic hepatocellular carcinoma actually turned out to be a fibrolamellar variant. It showed smears with moderate cellularity and singly dispersed malignant hepatocytes displaying frequent binucleation, prominent nucleoli, and abundant, granular, "oncocytic" cytoplasm associated with fragments of fibrous tissue (Fig. 5).
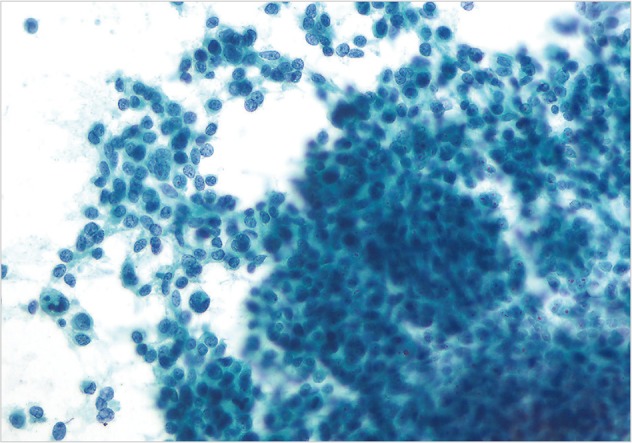
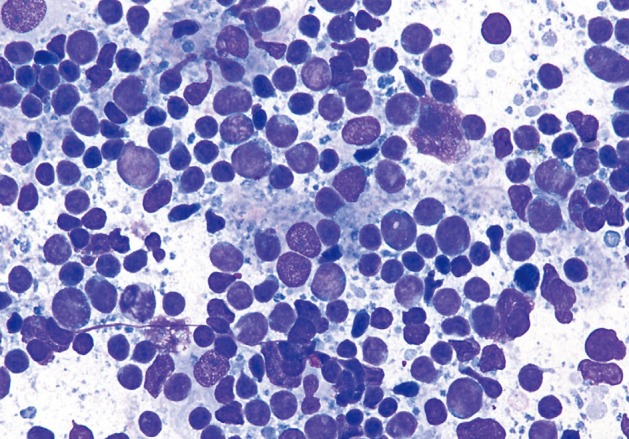
- The one case of metastatic melanoma was hypercellular with tissue fragments as well as singly dispersed malignant cells (Fig. 6). The tumor cells were relatively monotonous with round to oval often binucleated nuclei, and resembling an epithelioid neoplasm. The cells displayed single, prominent, eosinopohilic ("cherry red") nucleoli. The patient's history of metastatic melanoma was known at the time of FNA. The cases of non-Hodgkin lymphomas displayed hypercellular smears with monotonous-appearing, large lymphocytes (Fig. 7). The malignant cells often showed prominent nucleoli with background smears containing abundant lymphoglandular bodies, occasional tangible-body macrophages and karyorrhectic debris. A few cases displayed brisk mitotic activity.
- Follow-up clinical/histopathologic data
- Of those cases with surgical resection follow-up, one case diagnosed as metastatic pancreatic adenocarcinoma on FNA was later found to be of colonic origin. One of the metastatic carcinomas of unknown origin was proved to be a primary gynecologic malignancy and another case turned out to be a metastatic pancreatic endocrine tumor.
- The follow-up results are summarized in Table 2. Two of 4 cases called "atypical/suspicious for malignancy" on FNA and later confirmed by tissue studies to be malignant were included in the positive cytology group. The sensitivity, specificity, positive predictive value and negative predictive value in our study is 90%, 100%, 100%, and 75%, respectively.
RESULTS
- The pancreas is a retroperitoneal organ with close proximity to the duodenum, common bile duct, superior mesenteric artery, portal vein, transverse colon, spleen, stomach, and left lobe of the liver. The PPS is a zone of soft tissue between the pancreas and those aforementioned structures and contains lymph nodes, blood vessels, and nerves. Lesions of the PPS are rare but, when present, may lead to a mass effect causing clinical symptoms such as jaundice and abdominal pain. Current imaging modalities such as CT, ultrasound, and echoendoscopy are highly sensitive in detecting PPS lesions. However, they are limited in distinguishing between benign and malignant disease or primary pancreatic carcinomatous extension and metastatic disease. It is, therefore, important to have a tissue diagnosis to guide the appropriate clinical management. FNA is ideally suited to approach lesions in this anatomically difficult to access location of the retroperitoneum. It eliminates the need for open surgical biopsy or even radical resection with its associated morbidity and mortality.
- FNA of PPS is a rare specimen for cytopathologic interpretation. In a 16-year period from two large tertiary care institutions only 42 cases were located from thousands of abdominal aspirations. One reason might be the radiologic difficulty in separating peripancreatic lesions from true pancreatic masses.10 The pancreatic FNAs are mostly performed to rule out tumors, whereas the peripancreatic FNAs are done for preoperative staging of suspected pancreatic cancers to guide the proper management plan.8
- The differential diagnosis of a PPS lesion includes a broad spectrum of pathologic entities. Most of the lesions in our series were metastatic carcinomas (18/38, 47.4%). Primary pancreatic ductal adenocarcinoma represented nearly 1/3 of all metastatic cancers and 13.2% (5/18) of all PPS lesions in our series. Therefore, as expected, ductal adenocarcinoma of the pancreas is still the most likely diagnosis in a PPS FNA. Cancers developing in organs surrounding the PPS like the liver, kidneys and colon may also metastasize to the PPS, cytologically mimicking primary pancreatic carcinoma. Using EUS FNA for peripancreatic masses, Frazee et al.8 reported a variety of non-pancreatic tumors including cholangiocarcinoma, lymphoma, gastric adenocarcinoma, gallbladder carcinoma, paraganglioma, breast carcinoma, and leiomyosarcoma. In their series 57% of the results were pancreatic adenocarcinoma.9 An accurate clinical history of any known previous neoplasm often helps tremendously to narrow the list of possible diagnoses. In our series 11 patients had a prior history of malignancy and 6 of them had metastasis of the same cancer to the PPS. In doubtful cases, it is easier to compare the cytomorphology of possible metastasis to the original tumor to make the diagnosis. Additionally, it helps to select an appropriate immunoperoxidase panel to confirm the cytopathologic diagnosis.
- Peripancreatic lymph node involvement by lymphoma can be difficult to interpret, particularly when smears are composed of a mixed population of small and large lymphocytes mimicking a reactive process. Also, low-grade lymphoproliferative disorders composed of small lymphocytes cannot be reliably diagnosed by cytomorphology alone. Flow cytometry and immunoperoxidase studies are important adjuncts to establish an accurate diagnosis in these difficult specimens.11-14 Five non-Hodgkin lymphoma cases in our series had no prior history, and the PPS FNA was the initial diagnosis of lymphomatous involvement which was later confirmed by flow cytometry.
- Uncommonly, a PPS lesion is the initial presentation of an occult cancer. Seven of the 25 (28%) malignant cases in our study presented as occult metastasis. The cytomorphology of these cases was mostly poorly differentiated carcinoma. Employing immunoperoxidase studies can be very useful to confirm the cytomorphologic impression of the cancer origin. This is often tremendously helpful in guiding the clinicoradiologic search for the primary site of the tumor, especially when there is no radiological evidence of a primary tumor. In 5 of 10 cases when immunoperoxidase studies were performed in our series we were able to identify the primary site of metastatic cancers in the PPS. The often helpful immunoperoxidase stains in this regard are cytokeratins 7 and 20,15 DPC4 (expression is lost in 55% of pancreatic adenocarcinomas),16 CDX-2 and/or villin (usually positive in intestinal carcinomas, especially colonic),15 and markers like HMB-45/S-100 protein,15 TTF-1/napsin-A,17 HepPar-1,15 RCC/PAX-2,15 and inhibin (relatively specific for melanoma, carcinomas of the lung, liver, kidney, and the adrenals, respectively).15
- In conclusion, FNA of PPS masses is a rare occurrence. The majority of lesions are metastatic carcinomas from a variety of primary sites. Flow cytometry and immunoperoxidase studies are useful adjuncts to determine the tumor origin. The sensitivity of PPS aspiration for a malignant diagnosis is 90% with a positive predictive value of 100%.
DISCUSSION
- 1. Brand B, Pfaff T, Binmoeller KF, et al. Endoscopic ultrasound for differential diagnosis of focal pancreatic lesions, confirmed by surgery. Scand J Gastroenterol 2000; 35: 1221-1228. ArticlePubMed
- 2. de Roos WK, Welvaart K, Bloem JL, Hermans J. Assessment of resectability of carcinoma of the pancreatic head by ultrasonography and computed tomography: a retrospective analysis. Eur J Surg Oncol 1990; 16: 411-416. PubMed
- 3. Pasanen PA, Partanen KP, Pikkarainen PH, Alhava EM, Janatuinen EK, Pirinen AE. A comparison of ultrasound, computed tomography and endoscopic retrograde cholangiopancreatography in the differential diagnosis of benign and malignant jaundice and cholestasis. Eur J Surg 1993; 159: 23-29. PubMed
- 4. Snady H, Cooperman A, Siegel J. Endoscopic ultrasonography compared with computed tomography with ERCP in patients with obstructive jaundice or small peri-pancreatic mass. Gastrointest Endosc 1992; 38: 27-34. ArticlePubMed
- 5. Cahn M, Chang K, Nguyen P, Butler J. Impact of endoscopic ultrasound with fine-needle aspiration on the surgical management of pancreatic cancer. Am J Surg 1996; 172: 470-472. ArticlePubMed
- 6. Erickson RA, Garza AA. Impact of endoscopic ultrasound on the management and outcome of pancreatic carcinoma. Am J Gastroenterol 2000; 95: 2248-2254. ArticlePubMed
- 7. Di Stasi M, Lencioni R, Solmi L, et al. Ultrasound-guided fine needle biopsy of pancreatic masses: results of a multicenter study. Am J Gastroenterol 1998; 93: 1329-1333. ArticlePubMed
- 8. Frazee RC, Singh H, Erickson RA. Endoscopic ultrasound for peripancreatic masses. Am J Surg 1997; 174: 596-598. ArticlePubMed
- 9. Suits J, Frazee R, Erickson RA. Endoscopic ultrasound and fine needle aspiration for the evaluation of pancreatic masses. Arch Surg 1999; 134: 639-642. ArticlePubMed
- 10. Benning TL, Silverman JF, Berns LA, Geisinger KR. Fine needle aspiration of metastatic and hematologic malignancies clinically mimicking pancreatic carcinoma. Acta Cytol 1992; 36: 471-476. PubMed
- 11. Nicol TL, Silberman M, Rosenthal DL, Borowitz MJ. The accuracy of combined cytopathologic and flow cytometric analysis of fine-needle aspirates of lymph nodes. Am J Clin Pathol 2000; 114: 18-28. ArticlePubMed
- 12. Siebert JD, Weeks LM, List LW, et al. Utility of flow cytometry immunophenotyping for the diagnosis and classification of lymphoma in community hospital clinical needle aspiration/biopsies. Arch Pathol Lab Med 2000; 124: 1792-1799. ArticlePubMedPDF
- 13. Moriarty AT, Wiersema L, Snyder W, Kotylo PK, McCloskey DW. Immunophenotyping of cytologic specimens by flow cytometry. Diagn Cytopathol 1993; 9: 252-258. ArticlePubMed
- 14. Simsir A, Fetsch P, Stetler-Stevenson M, Abati A. Immunophenotypic analysis of non-Hodgkin's lymphomas in cytologic specimens: a correlative study of immunocytochemical and flow cytometric techniques. Diagn Cytopathol 1999; 20: 278-284. ArticlePubMed
- 15. Oien KA. Pathologic evaluation of unknown primary cancer. Semin Oncol 2009; 36: 8-37. ArticlePubMed
- 16. Tascilar M, Offerhaus GJ, Altink R, et al. Immunohistochemical labeling for the Dpc4 gene product is a specific marker for adenocarcinoma in biopsy specimens of the pancreas and bile duct. Am J Clin Pathol 2001; 116: 831-837. ArticlePubMed
- 17. Bishop JA, Sharma R, Illei PB. Napsin A and thyroid transcription factor-1 expression in carcinomas of the lung, breast, pancreas, colon, kidney, thyroid, and malignant mesothelioma. Hum Pathol 2010; 41: 20-25. ArticlePubMed
REFERENCES
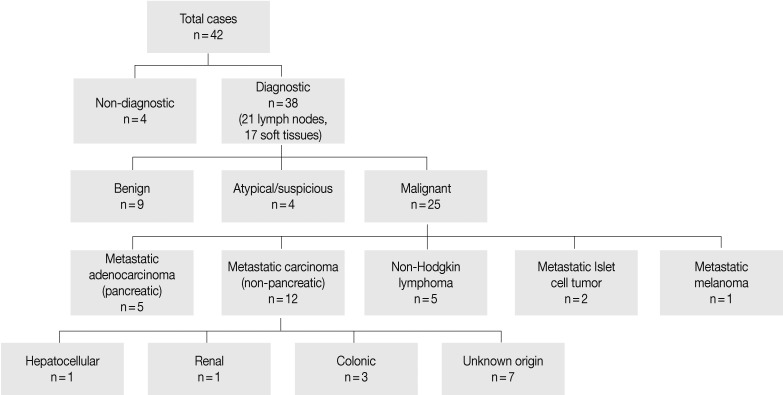
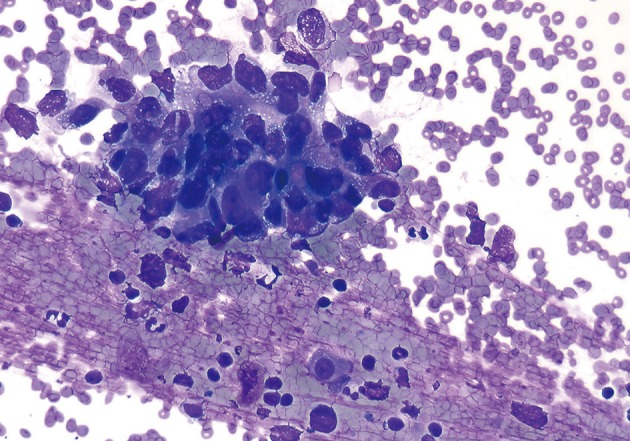
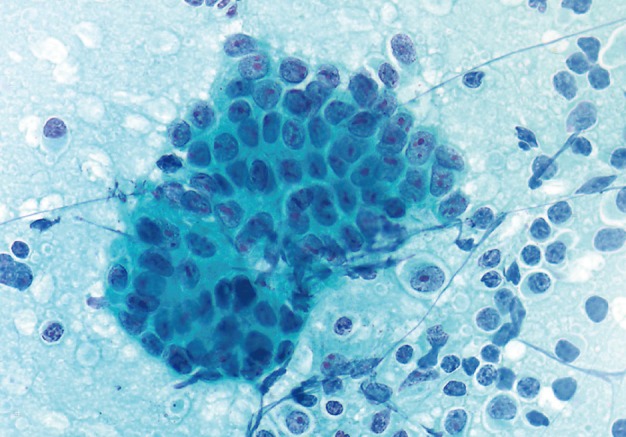
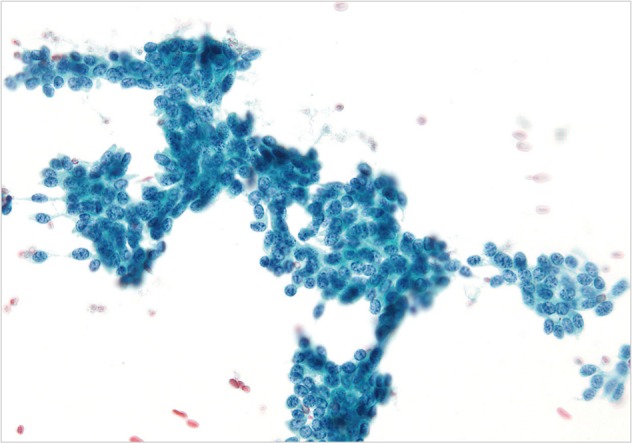
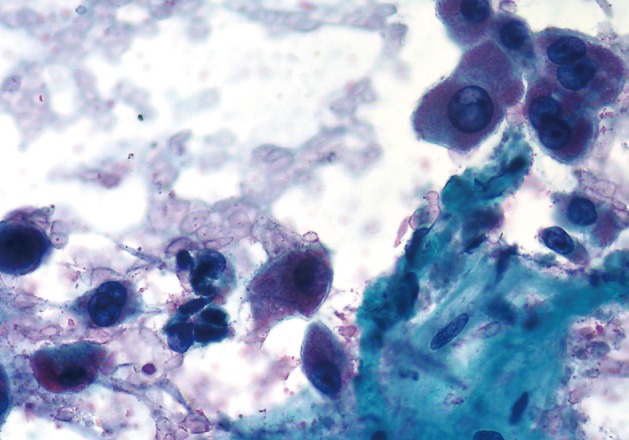

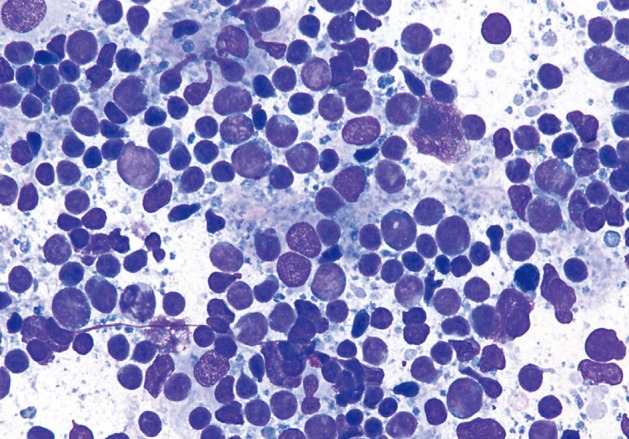
Figure & Data
References
Citations

 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure







Fig. 1
Fig. 2
Fig. 3
Fig. 4
Fig. 5
Fig. 6
Fig. 7
| Case No. | Age (yr) | Gender | History of previous malignancy | Clinical presentation | Radiological finding | Radiological differential diagnosis |
|---|---|---|---|---|---|---|
| 1 | 23 | F | Yes | Cancer follow-up | Mass of the pancreatic head, lymphadenopathy | Recurrent HCC |
| 2 | 38 | F | No | Abdominal pain | Lesion of the pancreatic body | Benign |
| 3 | 43 | F | Yes | Jaundice | Soft tissue density at the SMA | Primary vs metastatic neoplasm |
| 4 | 56 | F | No | Abdominal pain, vomiting | Peripancreatic cysts | Peripancreatitis |
| 5 | 59 | F | Yes | Pancreatic mass | Mass of the pancreatic head, diffuse lymphadenopathy | Lymphoma |
| 6 | 60 | F | No | Abdominal pain | Pancreatic mass, peripancreatic lymphadenopathy | Pancreatic primary neoplasm |
| 7 | 62 | F | No | Weight loss | Lymphadenopathy, suspicious pancreatic mass | Post surgery changes |
| 8 | 64 | F | No | Abdominal pain, abnormal LFTs | Diffuse abdominal lymphadenopathy, soft tissue mass in the peripancreas | Lymphoma |
| 9 | 64 | F | No | Not specified | No specific radiologic findings | |
| 10 | 67 | F | No | Abdominal pain | Peripancreatic lymphadenopathy, numerous liver masses | Carcinoma |
| 11 | 67 | F | Yes | Abdominal pain | Enlarged pancreatic head | Pancreatitis vs neoplasm |
| 12 | 67 | F | Yes | Jaundice | Mass of the pancreatic uncinate | Pancreatic primary neoplasm |
| 13 | 71 | F | No | Jaundice | Small periampullary mass | No evidence of malignancy |
| 14 | 71 | F | No | Abdominal pain, abnormal LFT | Pancreatic uncinate mass | Pancreatic adenocarcinoma |
| 15 | 72 | F | Yes | Guaiac+stool | Mass of the pancreatic head | Pancreatic primary neoplasm |
| 16 | 73 | F | Yes | Anemia | Peripancreatic mass | C/w metastatic RCC |
| 17 | 74 | F | No | Jaundice | Pancreatic mass, lymphadenopathy | Pancreatic primary neoplasm |
| 18 | 76 | F | No | Abdominal pain, weight loss | Duodenal mass involving the pancreas | Duodenal neoplasm |
| 19 | 78 | F | No | Abdominal pain | Pseudocyst | Pseudocyst |
| 20 | 80 | F | No | Weight loss | Mass of the pancreatic body | Lymphoma vs adenocarcinoma |
| 21 | 81 | F | No | Unstable angina | Pancreatic mass | Pancreatic primary neoplasm |
| 22 | 31 | M | No | Scrotal swelling, HIV positive, disseminated TB | Extensive peripancreatic lymphadenopathy with calcification, hepatomegaly | Lymphoma vs extensive TB |
| 23 | 31 | M | Yes | Lymphadenopathy | Peripancreatic lymphadenopathy | Recurrent lymphoma |
| 24 | 35 | M | No | Nausea, hepatosplenomegaly | Extensive peripancreatic lymphadenopathy, hepatosplenomegaly | Suspicious for lymphoma |
| 25 | 35 | M | Yes | Nausea, back pain | Peripancreatic fluid/mass, liver masses | Metastatic neoplasm |
| 26 | 43 | M | No | Not specified | No specific radiologic findings | |
| 27 | 47 | M | No | Abdominal pain | Retroperitoneal masses | Lymphoma vs sarcoma |
| 28 | 48 | M | No | Jaundice, back pain | Pancreatic mass, liver mass, peripancreatic lymphadenopathy | Pancreatic primary neoplasm |
| 29 | 51 | M | Yes | Lower extremity weakness, abnormal LFTs | Pancreatic head mass | Pancreatic primary neoplasm |
| 30 | 55 | M | No | Abdominal pain, weight loss | Abnormal appearance of pancreatic head and body, peripancreatic lymphadenopathy | Carcinoma |
| 31 | 55 | M | No | Abdominal pain, night sweats | Peripancreatic lymphadenopathy | |
| 32 | 57 | M | No | Abdominal pain | Pancreatic mass, peripancreatic lymphadenopathy | Pancreatic neoplasm |
| 33 | 58 | M | No | Jaundice | Liver mass, peripancreatic lymphadenopathy | Suspicious for metastatic HCC |
| 34 | 64 | M | No | Jaundice | Mass of the pancreatic head | Chronic pancreatitis |
| 35 | 64 | M | No | Nonspecific | Peripancreatic lymphadenopathy | Lymphoma |
| 36 | 65 | M | No | ESRD for transplant | Pancreatic mass | Suspicious for pancreatic primary |
| 37 | 65 | M | Yes | Hematuria | Pancreatic mass, lymphadenopathy | Pancreatic adenocarcinoma |
| 38 | 66 | M | No | Not specified | No detailed radiologic findings | |
| 39 | 73 | M | No | Not specified | No detailed radiologic findings | |
| 40 | 78 | M | Yes | Cancer follow-up | Peripancreatic lymphadenopathy | Lymphoma vs metastasis |
| 41 | 81 | M | No | Not specified | No detailed radiologic findings | |
| 42 | 83 | M | No | Abdominal pain | Peripancreatic mass | Lymphoma vs carcinoid vs sclerosing mesenteritis |
| Follow-up Positive | Follow-up Negative | Total | ||
|---|---|---|---|---|
| Cytology positive | 26 | 0 | 26 | PPV 100% |
| Cytology negative | 3 | 9 | 12 | NPV 75% |
| Total | 29 | 9 | 38 | |
| Sensitivity 90% | Specificity 100% |
F, female; HCC, hepatocellular carcinoma; SMA, superior mesenteric artery; LFTs, liver function tests; RCC, renal cell carcinoma; M, male; HIV, human immunodeficiency virus; TB, tuberculosis; ESRD, end stage renal disease; vs, versus; c/w, consistent with.
PPV, positive predictive value; NPV, negative predictive value.

 E-submission
E-submission