Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 47(5); 2013 > Article
-
Original Article
Clinicopathological Analysis of Hepatocellular Adenoma According to New Bordeaux Classification: Report of Eight Korean Cases - Hyunchul Kim, Ja-June Jang1, Dong-Sik Kim2, Beom Woo Yeom3, Nam Hee Won3
-
Korean Journal of Pathology 2013;47(5):411-417.
DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.5.411
Published online: October 25, 2013
Department of Pathology, Gachon University Gil Medical Center, Incheon, Korea.
1Department of Pathology, Seoul National University College of Medicine, Seoul, Korea.
2Division of and Hepato-bilio-pancreas Surgery and Liver Transplantation, Department of Surgery, Korea University College of Medicine, Seoul, Korea.
3Department of Pathology, Korea University College of Medicine, Seoul, Korea.
- Corresponding Author: Nam Hee Won, M.D. Department of Pathology, Korea University College of Medicine, 73 Inchon-ro, Seongbuk-gu, Seoul 136-705, Korea. Tel: +82-2-920-6142, Fax: +82-2-953-3130, nhw@korea.ac.kr
© 2013 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations

- Perinatal Management of Hepatic Adenomas
Megan A. Nocita, Carla W. Brady, Jeffrey A. Kuller, Luke A. Gatta
Obstetrical & Gynecological Survey.2024; 79(12): 735. CrossRef - Relevance of morphological features for hepatocellular adenoma classification in pathology practice
Carla Henriques Agostini, Osmar Damasceno Ribeiro, Arlete Fernandes, Adriana Caroli-Bottino, Vera Lucia Pannain
Surgical and Experimental Pathology.2020;[Epub] CrossRef - The molecular functions of hepatocyte nuclear factors – In and beyond the liver
Hwee Hui Lau, Natasha Hui Jin Ng, Larry Sai Weng Loo, Joanita Binte Jasmen, Adrian Kee Keong Teo
Journal of Hepatology.2018; 68(5): 1033. CrossRef - Hepatocellular adenoma: Classification, variants and clinical relevance
Paulette Bioulac-Sage, Christine Sempoux, Charles Balabaud
Seminars in Diagnostic Pathology.2017; 34(2): 112. CrossRef - A Limited Immunohistochemical Panel Can Subtype Hepatocellular Adenomas for Routine Practice
Brent K. Larson, Maha Guindi
American Journal of Clinical Pathology.2017; 147(6): 557. CrossRef - Hepatocellular Neoplasms Arising in Association With Androgen Use
Sounak Gupta, Bita V. Naini, Richard Munoz, Rondell P. Graham, Benjamin R. Kipp, Michael S. Torbenson, Taofic Mounajjed
American Journal of Surgical Pathology.2016; 40(4): 454. CrossRef - Pigmented hepatocellular adenomas have a high risk of atypia and malignancy
Taofic Mounajjed, Saba Yasir, Patrice A Aleff, Michael S Torbenson
Modern Pathology.2015; 28(9): 1265. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure



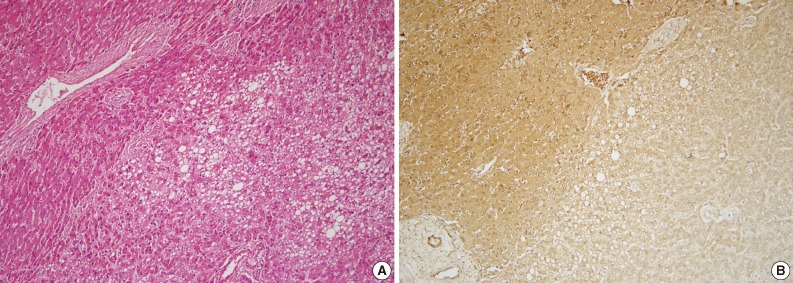
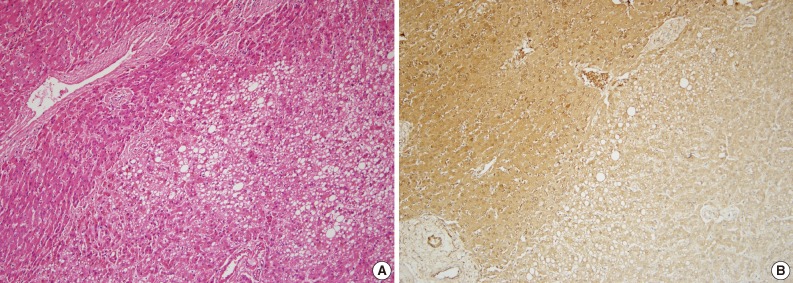
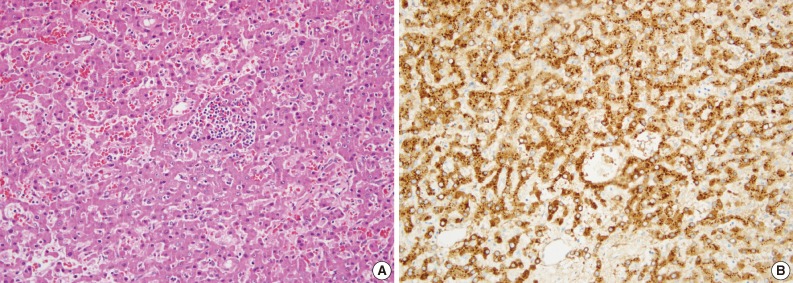
Fig. 1
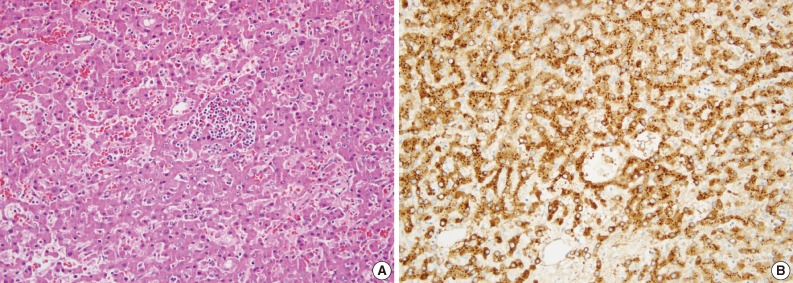
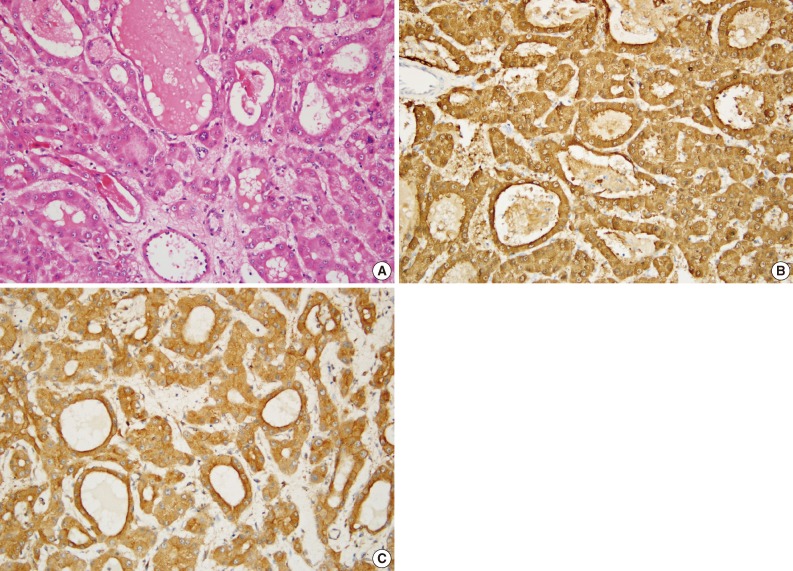
Fig. 2
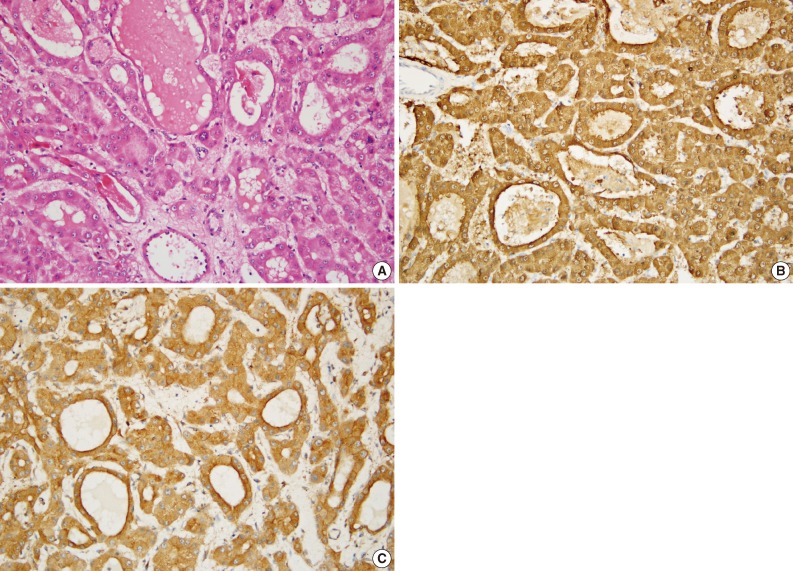
Fig. 3
| Subtype | Case | Sex | Age (yr) | BMI | Alcohol consumption | No. of nodules | Size (cm) | Intratumoral hemorrhage |
|---|---|---|---|---|---|---|---|---|
| H-HCA | A | F | 56 | 20.312 | No | 1 | 3.0 | - |
| B | M | 13 | N/A | No | > 5 | 5.5 |
+ | |
| C | F | 27 | 18.614 | No | 1 | 11.0 | + | |
| I-HCA | D | M | 28 | 27.811 | No | 1 | 4.6 | + |
| E | F | 20 | 20.889 | No | 3 | 10.0 |
+ | |
| F | M | 27 | N/A | N/A | 1 | 5.5 | + | |
| G | M | 35 | 25.909 | No | 1 | 4.5 | - | |
| β-HCA | H | F | 17 | N/A | No | 1 | 11.0 | + |
| Subtype | Case | Steatosis (%) | Sinusoidal dilation | Inflammatory infiltrate | Cytological atypia | Acinar pattern | Thick walled artery | Telangiectasia | Peliosis |
|---|---|---|---|---|---|---|---|---|---|
| H-HCA | A | 30-40 | - | - | - | - | - | + | - |
| B | 20 | - | - | - | - | - | - | - | |
| C | 0 | - | - | + | - | + | + | - | |
| I-HCA | D | 5 | + | + | + | - | + | - | - |
| E | 5 | + | + | - | - | + | + | - | |
| F | 0 | + | + | - | - | + | + | + | |
| G | 0 | + | + | + | - | + | + | - | |
| β-HCA | H | 0 | + | - | + | + | + | - | - |
| Subtype | Case | L-FABP | β-Catenin | SAA | Glutamine synthetase | CD3 | CD20 |
|---|---|---|---|---|---|---|---|
| H-HCA | A | - | - | - | + | + | - |
| B | - | - | - | - | + | + | |
| C | - | - | - | + | + | - | |
| I-HCA | D | + | - | + | - | + | + |
| E | + | - | + | + | + | + | |
| F | + | - | + | + | + | + | |
| G | + | - | + | - | + | + | |
| β-HCA | H | + | - | + | + | + | - |
BMI, body mass index; H-HCA, hepatocyte nuclear factor 1α-mutated hepatocellular adenoma; F, female; M, male; N/A, not available; I-HCA, inflammatory hepatocellular adenoma; β-HCA, β-catenin-mutated hepatocellular adenoma. The largest mass.
H-HCA, hepatocyte nuclear factor 1α-mutated hepatocellular adenoma; I-HCA, inflammatory hepatocellular adenoma; β-HCA, β-catenin-mutated hepatocellular adenoma
L-FABP, liver fatty acid binding protein; SAA, serum amyloid A; H-HCA, hepatocyte nuclear factor 1α-mutated hepatocellular adenoma; I-HCA, inflammatory hepatocellular adenoma; β-HCA, β-catenin-mutated hepatocellular adenoma.

 E-submission
E-submission