Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 47(5); 2013 > Article
-
Original Article
Histologic Variations and Immunohistochemical Features of Metastatic Clear Cell Renal Cell Carcinoma - Cheol Lee1, Jeong-Whan Park1, Ja Hee Suh1, Kyung Han Nam1, Kyung Chul Moon1,2
-
Korean Journal of Pathology 2013;47(5):426-432.
DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.5.426
Published online: October 25, 2013
1Department of Pathology, Seoul National University College of Medicine, Seoul, Korea.
2Kidney Research Institute, Medical Research Center, Seoul National University College of Medicine, Seoul, Korea.
- Corresponding Author: Kyung Chul Moon, M.D. Department of Pathology, Kidney Research Institute, Medical Research Center, Seoul National University College of Medicine, 103 Daehak-ro, Jongno-gu, Seoul 110-799, Korea. Tel: +82-2-2072-1767, Fax: +82-2-743-5530, blue7270@snu.ac.kr
© 2013 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Due to advancements in treatment of metastatic and advanced renal cell carcinoma (RCC), it has become increasingly important to diagnose metastatic RCC and the specific subtype. In this study, we investigated the diverse histologic features of metastatic clear cell renal cell carcinoma (CCRCC) cases in comparison with corresponding primary lesions.
-
Methods
- We identified 119 metastatic CCRCC cases from 81 corresponding primary lesions diagnosed between 1995 and 2010 and evaluated the diverse histologic and immunohistochemical features of these lesions.
-
Results
- A total of 44 primary lesions (54.3%) had a non-clear cell component in addition to a typical clear cell component. Of the 119 metastatic lesions, 63 lesions (52.9%) contained a non-clear cell component, and 29 metastatic lesions were composed of a non-clear cell component only. Rhabdoid features were the most frequent non-clear cell histology among the metastatic lesions. Metastatic CCRCCs mainly showed positive CD10 and epithelial membrane antigen staining and negative cytokeratin 7 staining.
-
Conclusions
- Metastatic CCRCC commonly showed a variety of histologic features. If there is a difficulty to diagnose metastatic CCRCC due to a variety of histologic features or small biopsy specimen, histologic review of the primary lesion and immunohistochemical analysis can help determine the correct diagnosis.
- We identified cases of metastatic CCRCC and corresponding primary lesions diagnosed at Seoul National University Hospital between 1995 and 2010. A total of 119 metastatic CCRCC cases from 81 primary CCRCCs were available. With the exception of one metastatic specimen obtained by needle biopsy, all cases were obtained by excision. We collected clinical and pathological information from electronic medical records and pathologic reports and reviewed hematoxylin and eosin-stained slides for all samples as well as all available immunohistochemical slides. The RCC subtypes were reclassified according to the 2004 World Health Organization (WHO) classification.7 Additionally, we retrospectively graded tumors as grade I to IV according to the Fuhrman nuclear grading system. This study was approved by the Institutional Review Board (IRB) of Seoul National University Hospital. Histologic and immunohistochemical differences between primary and metastatic lesions were statistically analyzed using Student's t-test, and the statistics program SPSS ver. 21.0 (IBM Co., Armonk, NY, USA) was used for statistical calculations.
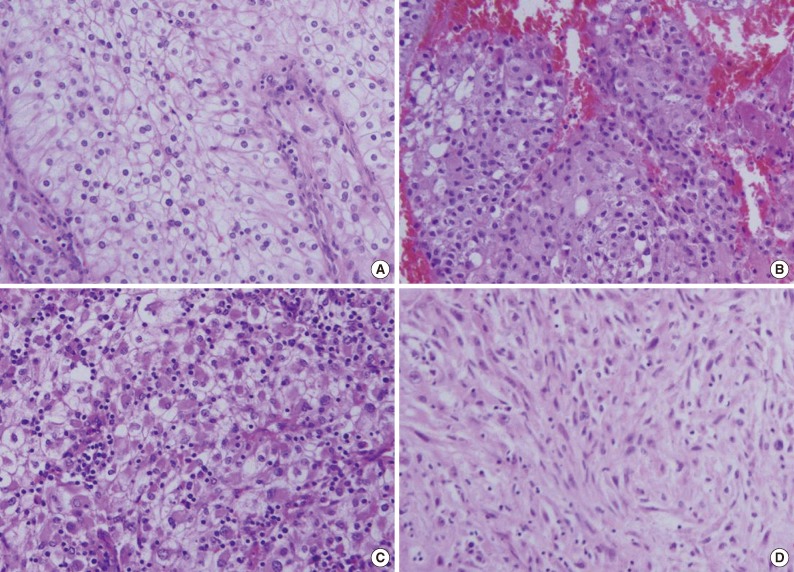
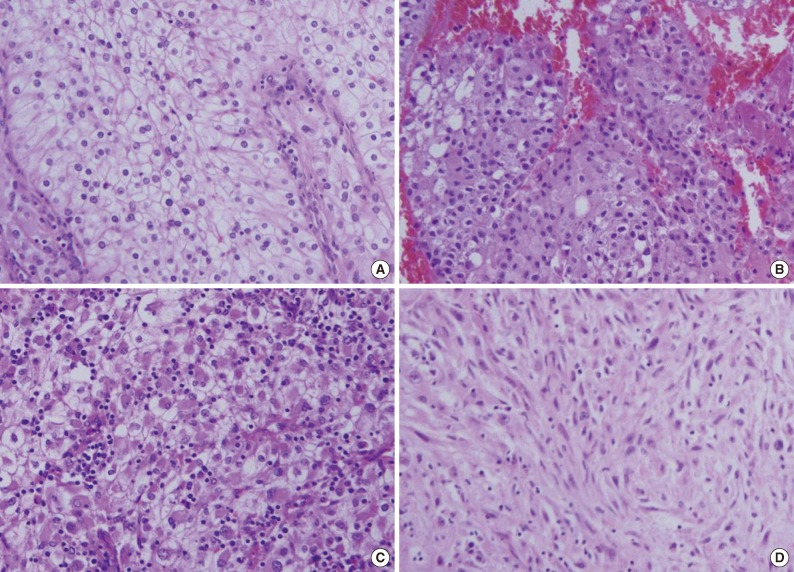
- In the 81 primary CCRCC cases and 119 cases of metastatic CCRCCs, we observed several non-clear cell histologic features. Among those features, we determined relatively common fea tures such as eosinophilic cytoplasm, rhabdoid features, and sarcomatoid differentiation (Fig. 1).
MATERIALS AND METHODS
- Basic clinicopathologic characteristics
- A total of 81 patients were included in this study, including 64 (79.0%) male and 17 (21.0%) female patients. The age at diagnosis ranged from 31 to 80 years, and the mean and median ages at diagnosis were 55.9 and 57 years, respectively. The distribution of the Fuhrman nuclear grading was no cases of grade I (0%), 19 cases of grade II (23.5%), 48 cases of grade III (59.3%), and 14 cases of grade IV (17.3%). According to prognostic grouping of the American Joint Committee on Cancer 7th edition, our cases had a distribution with the following pattern: 25 cases of stage I (30.9%), 12 cases of stage II (14.8%), 16 cases of stage III (19.8%), and 28 cases of stage IV (34.6%) (Table 1).
- Of the 119 metastatic lesions, 49 cases (41.2%) were pulmonary metastasis and 26 (21.8%) were bone metastasis. Other frequent metastatic sites included soft tissue (14 cases, 11.8%), skin (6 cases, 5.0%), pancreas (5 cases, 4.2%), liver (4 cases, 3.4%), and adrenal gland (4 cases, 3.4%) (Table 2).
- Histologic variations of primary and metastatic lesions
- Overall histologic features of primary and metastatic lesions are described in Table 3. All 81 primary CCRCCs contained a variable proportion of typical clear cell component, and some cases had only a focal area of clear cell component. There were 37 primary lesions (45.7%) composed of only typical clear cell component, while the remaining 44 cases (54.3%) included a non-clear cell component in addition to typical clear cell area. Of the 119 metastatic lesions, 56 lesions (47.1%) were composed of only typical clear cells, and the remaining 63 lesions (52.9%) included a non-clear cell component. Among the 63 metastatic lesions with a non-clear cell component, 29 (24.4%) were composed of a non-clear cell component only without a typical clear cell area. Rhabdoid features were the most frequent non-clear cell histology in the metastatic lesions, and three metastatic lesions were exclusively composed of unclassifiable, non-characteristic histology. The frequencies of each histologic feature between primary and metastatic lesions were not statistically different (Table 4).
- Non-clear cell components observed in metastatic lesions were also present in the primary lesions. In 51 of 63 (81.0%) metastatic lesions with a non-clear cell component, the corresponding primary lesion showed the same histologic features. However, the remaining 12 metastatic tumors (19.0%) had a non-clear cell component not found in the corresponding primary tumor.
- Of the 63 metastatic lesions with a non-clear cell component, 17 cases had multiple metastatic lesions. The histologic variations of multiple metastatic lesions are described in Table 5. Among 17 multiple metastasis cases, four cases (case nos. 12, 16, 69, and 73) revealed different histology in each metastatic lesion (Table 5).
- Immunohistochemical results
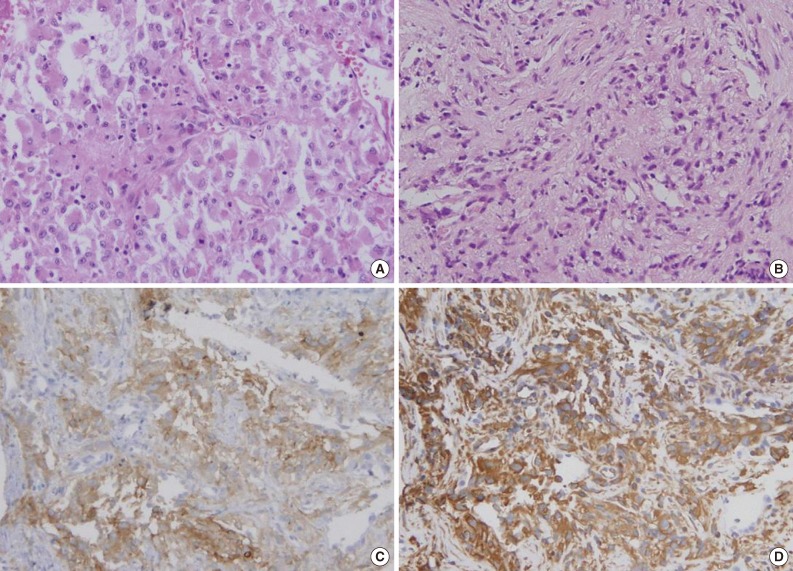
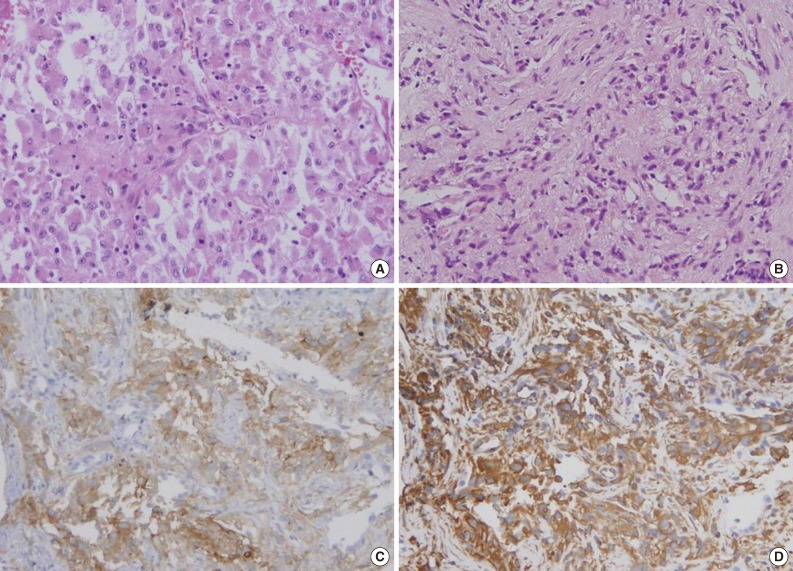
- Immunohistochemical staining was performed in 21 primary lesions and 51 metastatic lesions. We reviewed all immunohistochemical staining and classified results as positive or negative. The results are shown in Table 6. Staining for CD10 and epithelial membrane antigen (EMA) was positive in most primary and metastatic CCRCCs. Renal cell carcinoma marker (RCC Ma), pan-cytokeratin (CK), vimentin (VT), and PAX2 showed positive staining in approximately 50% of the primary and metastatic CCRCCs. Cytokeratin 7 (CK7) showed positive staining in only one case of metastatic CCRCC, and cytokeratin 20 (CK20) showed negative staining in all stained tumors. Immunohistochemical results between primary and metastatic lesions were not statistically different (Table 6).
- Diagnosis of the three metastatic lesions exclusively composed of non-characteristic, unclassifiable histology was difficult, but immunohistochemical staining aided in making the correct diagnosis (Fig. 2).
RESULTS
- In this study, we found that metastatic CCRCCs frequently showed non-typical histologic features such as eosinophilic cytoplasm, rhabdoid features, and sarcomatoid differentiation, which were features also found in corresponding primary lesions. These non-typical histologic variations occasionally made the diagnosis of metastatic RCC difficult, and in these cases, histologic review of the primary lesion is important for the diagnosis of metastatic RCC.
- Abel et al.20,21 claimed that preoperative biopsy specimens were limited in their ability to identify histologic subtype (especially non-clear cell type), Fuhrman nuclear grade, or sarcomatoid differentiation. They analyzed needle biopsy specimens from primary or metastatic lesions from patients with metastatic RCC compared with cytoreductive nephrectomy specimens.20 When surgical resection is not available, they recommend obtaining multiple samples from primary sites to obtain more precise information about the tumor.20 The studies involved all subtypes of RCC samples, but this study only included CCRCC samples. We also experienced the limitation of small biopsy specimens showing only non-typical histologic features. However, because we analyzed mainly resected specimens, the difficulties of diagnosing metastatic CCRCC were thought to be due to non-typical histologic features, which tended to appear frequently in metastatic CCRCCs, rather than small specimen size. As mentioned earlier, 29 of 119 (24.4%) metastatic CCRCC cases showed non-clear cell morphology only, while all 81 primary CCRCC cases contained a typical clear cell area.
- The rhabdoid cells in RCCs have a high nuclear grade and are associated with a high nuclear grade of a non-rhabdoid RCC component. Additionally, the presence of rhabdoid features in RCC is related to advanced pathologic stage. These associations suggest that identification of the rhabdoid feature in RCC likely portends a poor outcome.12 One study suggests that RCC with rhabdoid features is a highly aggressive neoplasm with a malignant behavior that may be due to the high activity of cell proliferation of the rhabdoid area.13 Another study suggests that rhabdoid areas show higher immunohistochemical p53 positivity than non-rhabdoid areas in the same tumors.15 Molecular genetic studies in a few cases have shown concordant loss of chromosome 3p and a VHL gene mutation in rhabdoid and clear cells from the same case, suggesting divergent differentiation from the same clone.22 Rhabdoid RCC is associated with sarcomatoid RCC in a significant number (22%) of cases.22 In this study, rhabdoid features were observed in approximately 30% of metastatic CCRCCs, and this frequency is quite higher than in primary lesions of RCC.12-16 This finding also suggests that rhabdoid features are associated with a poor prognosis.
- Several studies have suggested that most primary and metastatic CCRCCs are positive for CD10.23-27 These studies have shown that the immunohistochemical results of RCC Ma, CK, EMA, and VT staining were variable but have relatively high positivity in primary and metastatic CCRCCs.23-28 Additionally, these studies reported low positivity of CK7 and negativity of CK20 in primary CCRCCs.23 Although we investigated 21 primary lesions and 51 metastatic lesions with immunohistochemical staining, our findings were similar to those of the previous study. The positivity of CD10 and EMA and negativity of CK7 suggested metastatic CCRCC, which suggests that CD10, EMA, and CK7 may be useful makers for diagnosis of metastatic CCRCC. Also, recent studies have proposed the utility of PAX-2 or PAX-8 for the diagnosis of metastatic RCC.19,27,29,30
- In general, it is not difficult to diagnose metastatic RCC and to determine the subtype of the primary lesion from a metastatic specimen. However, it can be difficult in some cases, especially when small needle biopsy specimens are utilized. During the pathologic diagnosis of a lesion thought to be metastatic RCC, histologic variations of metastatic CCRCC such as eosinophilic cytoplasm, rhabdoid features, or sarcomatoid differentiation must be considered. It is also important to review the histology of the primary lesion, if possible. If metastatic RCC cannot be diagnosed by routine hematoxylin and eosin slides only, immunohistochemical staining may be helpful.
- In conclusion, metastatic CCRCC commonly showed a variety of histologic features, including eosinophilic cytoplasm, rhabdoid features, or sarcomatoid differentiation. If there is a difficulty to diagnose metastatic CCRCC because of a variety of histologic features or small biopsy specimens, histologic review of the primary lesion and immunohistochemical analysis can help determine the correct diagnosis.
DISCUSSION
Acknowledgments
Acknowledgments
- 1. Ljungberg B, Alamdari FI, Stenling R, Roos G. Prognostic significance of the Heidelberg classification of renal cell carcinoma. Eur Urol 1999; 36: 565-569. ArticlePubMed
- 2. Lam JS, Leppert JT, Figlin RA, Belldegrun AS. Surveillance following radical or partial nephrectomy for renal cell carcinoma. Curr Urol Rep 2005; 6: 7-18. ArticlePubMedPDF
- 3. Chow WH, Devesa SS, Warren JL, Fraumeni JF Jr. Rising incidence of renal cell cancer in the United States. JAMA 1999; 281: 1628-1631. ArticlePubMed
- 4. Reeves DJ, Liu CY. Treatment of metastatic renal cell carcinoma. Cancer Chemother Pharmacol 2009; 64: 11-25. ArticlePubMedPDF
- 5. Tamaskar I, Pili R. Update on novel agents in renal cell carcinoma. Expert Rev Anticancer Ther 2009; 9: 1817-1827. ArticlePubMed
- 6. Di Lorenzo G, Buonerba C, Biglietto M, et al. The therapy of kidney cancer with biomolecular drugs. Cancer Treat Rev 2010; 36(Suppl 3):S16-S20. ArticlePubMed
- 7. Eble JN, Sauter G, Epstein JI, Sesterhenn IA. Pathology and genetics of tumours of the urinary system and male genital organs. Lyon: IARC Press, 2004; 12-39.
- 8. Humphrey PA. Sarcomatoid renal cell carcinoma. J Urol 2012; 188: 601-602. ArticlePubMed
- 9. Cheville JC, Lohse CM, Zincke H, et al. Sarcomatoid renal cell carcinoma: an examination of underlying histologic subtype and an analysis of associations with patient outcome. Am J Surg Pathol 2004; 28: 435-441. PubMed
- 10. Mian BM, Bhadkamkar N, Slaton JW, et al. Prognostic factors and survival of patients with sarcomatoid renal cell carcinoma. J Urol 2002; 167: 65-70. ArticlePubMed
- 11. Shuch B, Bratslavsky G, Shih J, et al. Impact of pathological tumour characteristics in patients with sarcomatoid renal cell carcinoma. BJU Int 2012; 109: 1600-1606. ArticlePubMedPMC
- 12. Gökden N, Nappi O, Swanson PE, et al. Renal cell carcinoma with rhabdoid features. Am J Surg Pathol 2000; 24: 1329-1338. ArticlePubMed
- 13. Kuroiwa K, Kinoshita Y, Shiratsuchi H, et al. Renal cell carcinoma with rhabdoid features: an aggressive neoplasm. Histopathology 2002; 41: 538-548. ArticlePubMedPDF
- 14. Chapman-Fredricks JR, Herrera L, Bracho J, et al. Adult renal cell carcinoma with rhabdoid morphology represents a neoplastic dedifferentiation analogous to sarcomatoid carcinoma. Ann Diagn Pathol 2011; 15: 333-337. ArticlePubMed
- 15. Leroy X, Zini L, Buob D, Ballereau C, Villers A, Aubert S. Renal cell carcinoma with rhabdoid features: an aggressive neoplasm with overexpression of p53. Arch Pathol Lab Med 2007; 131: 102-106. ArticlePubMedPDF
- 16. Shannon B, Stan Wisniewski Z, Bentel J, Cohen RJ. Adult rhabdoid renal cell carcinoma. Arch Pathol Lab Med 2002; 126: 1506-1510. ArticlePubMedPDF
- 17. Weeks DA, Beckwith JB, Mierau GW, Luckey DW. Rhabdoid tumor of kidney: a report of 111 cases from the National Wilms' Tumor Study Pathology Center. Am J Surg Pathol 1989; 13: 439-458. PubMed
- 18. Truong LD, Shen SS. Immunohistochemical diagnosis of renal neoplasms. Arch Pathol Lab Med 2011; 135: 92-109. ArticlePubMedPDF
- 19. Sangoi AR, Karamchandani J, Kim J, Pai RK, McKenney JK. The use of immunohistochemistry in the diagnosis of metastatic clear cell renal cell carcinoma: a review of PAX-8, PAX-2, hKIM-1, RCCma, and CD10. Adv Anat Pathol 2010; 17: 377-393. PubMed
- 20. Abel EJ, Carrasco A, Culp SH, et al. Limitations of preoperative biopsy in patients with metastatic renal cell carcinoma: comparison to surgical pathology in 405 cases. BJU Int 2012; 110: 1742-1746. ArticlePubMed
- 21. Abel EJ, Culp SH, Matin SF, et al. Percutaneous biopsy of primary tumor in metastatic renal cell carcinoma to predict high risk pathological features: comparison with nephrectomy assessment. J Urol 2010; 184: 1877-1881. ArticlePubMedPMC
- 22. Humphrey PA. Renal cell carcinoma with rhabdoid features. J Urol 2011; 186: 675-676. ArticlePubMed
- 23. Allory Y, Bazille C, Vieillefond A, et al. Profiling and classification tree applied to renal epithelial tumours. Histopathology 2008; 52: 158-166. ArticlePubMed
- 24. Liu L, Qian J, Singh H, Meiers I, Zhou X, Bostwick DG. Immunohistochemical analysis of chromophobe renal cell carcinoma, renal oncocytoma, and clear cell carcinoma: an optimal and practical panel for differential diagnosis. Arch Pathol Lab Med 2007; 131: 1290-1297. ArticlePubMedPDF
- 25. Skinnider BF, Folpe AL, Hennigar RA, et al. Distribution of cytokeratins and vimentin in adult renal neoplasms and normal renal tissue: potential utility of a cytokeratin antibody panel in the differential diagnosis of renal tumors. Am J Surg Pathol 2005; 29: 747-754. PubMed
- 26. Avery AK, Beckstead J, Renshaw AA, Corless CL. Use of antibodies to RCC and CD10 in the differential diagnosis of renal neoplasms. Am J Surg Pathol 2000; 24: 203-210. ArticlePubMed
- 27. Rivera AL, Takei H, Zhai J, Shen SS, Ro JY, Powell SZ. Useful immunohistochemical markers in differentiating hemangioblastoma versus metastatic renal cell carcinoma. Neuropathology 2010; 30: 580-585. ArticlePubMed
- 28. Bakshi N, Kunju LP, Giordano T, Shah RB. Expression of renal cell carcinoma antigen (RCC) in renal epithelial and nonrenal tumors: diagnostic implications. Appl Immunohistochem Mol Morphol 2007; 15: 310-315. PubMed
- 29. Ozcan A, de la Roza G, Ro JY, Shen SS, Truong LD. PAX2 and PAX8 expression in primary and metastatic renal tumors: a comprehensive comparison. Arch Pathol Lab Med 2012; 136: 1541-1551. ArticlePubMedPDF
- 30. Sharma SG, Gokden M, McKenney JK, et al. The utility of PAX-2 and renal cell carcinoma marker immunohistochemistry in distinguishing papillary renal cell carcinoma from nonrenal cell neoplasms with papillary features. Appl Immunohistochem Mol Morphol 2010; 18: 494-498. ArticlePubMed
REFERENCES
| Parameter | No. (%) (n = 81) | |
|---|---|---|
| Sex | Male | 64 (79.0) |
| Female | 17 (21.0) | |
| Fuhrman grade | I | 0 (0) |
| II | 19 (23.5) | |
| III | 48 (59.3) | |
| IV | 14 (17.3) | |
| Stage | I | 25 (30.9) |
| II | 12 (14.8) | |
| III | 16 (19.8) | |
| IV | 28 (34.6) |
Figure & Data
References
Citations

- Sarcomatoid and Rhabdoid Renal Cell Carcinoma
Adebowale J. Adeniran, Brian Shuch, Peter A. Humphrey
American Journal of Surgical Pathology.2024; 48(7): e65. CrossRef - Emerging Antibody-Drug Conjugate Therapies and Targets for Metastatic Renal Cell Carcinoma
Harrison C. Gottlich, Reza Nabavizadeh, Mihai Dumbrava, Rodrigo Rodrigues Pessoa, Ahmed M. Mahmoud, Ishita Garg, Jacob Orme, Brian A. Costello, John Cheville, Fabrice Lucien
Kidney Cancer.2023; 7(1): 161. CrossRef - Painful, bleeding fingertip papule
Jane Gay, Sarah Simpson, Patrick Rush, Alex Holliday
JAAD Case Reports.2022; 21: 130. CrossRef - Development and initial clinical testing of a multiplexed circulating tumor cell assay in patients with clear cell renal cell carcinoma
Rory M. Bade, Jennifer L. Schehr, Hamid Emamekhoo, Benjamin K. Gibbs, Tamara S. Rodems, Matthew C. Mannino, Joshua A. Desotelle, Erika Heninger, Charlotte N. Stahlfeld, Jamie M. Sperger, Anupama Singh, Serena K. Wolfe, David J. Niles, Waddah Arafat, John
Molecular Oncology.2021; 15(9): 2330. CrossRef - Laparoscopic cytoreductive nephrectomy and adrenalectomy for metachronous RCC metastases—Case report
Bogdan Petrut, Cristina Eliza Bujoreanu, Vasile Vlad Hardo, Adrian Barbos, Bogdan Fetica
International Journal of Surgery Case Reports.2020; 74: 268. CrossRef - Does CARMENA mark the end of cytoreductive nephrectomy for metastatic renal cell carcinoma?
Steven L. Chang, Toni K. Choueiri, Lauren C. Harshman
Urologic Oncology: Seminars and Original Investigations.2019; 37(8): 525. CrossRef - Metastatic TFE3-overexpressing renal clear cell carcinoma with dense granules: a histological, immunohistochemical, and ultrastructural study
Shoujun Chen, Elba A. Turbat-Herrera, Guillermo A. Herrera, Meghna Chadha, Rodney E. Shackelford, Eric X. Wei
Ultrastructural Pathology.2018; 42(4): 369. CrossRef - The Clinical Activity of PD-1/PD-L1 Inhibitors in Metastatic Non–Clear Cell Renal Cell Carcinoma
Rana R. McKay, Dominick Bossé, Wanling Xie, Stephanie A.M. Wankowicz, Abdallah Flaifel, Raphael Brandao, Aly-Khan A. Lalani, Dylan J. Martini, Xiao X. Wei, David A. Braun, Eliezer Van Allen, Daniel Castellano, Guillermo De Velasco, J. Connor Wells, Daniel
Cancer Immunology Research.2018; 6(7): 758. CrossRef - Implication of PHF2 Expression in Clear Cell Renal Cell Carcinoma
Cheol Lee, Bohyun Kim, Boram Song, Kyung Chul Moon
Journal of Pathology and Translational Medicine.2017; 51(4): 359. CrossRef - Pulmonary metastasectomy from renal cell carcinoma including 3 cases with sarcomatoid component
Tsuyoshi Ueno, Motohiro Yamashita, Shigeki Sawada, Ryujiro Sugimoto, Noriko Nishijima, Yoshifumi Sugawara, Iku Ninomiya
General Thoracic and Cardiovascular Surgery.2016; 64(3): 149. CrossRef - Are primary renal cell carcinoma and metastases of renal cell carcinoma the same cancer?
Aleksandra Semeniuk-Wojtaś, Rafał Stec, Cezary Szczylik
Urologic Oncology: Seminars and Original Investigations.2016; 34(5): 215. CrossRef - Concordance of Pathologic Features Between Metastatic Sites and the Primary Tumor in Surgically Resected Metastatic Renal Cell Carcinoma
Sarah P. Psutka, John C. Cheville, Brian A. Costello, Suzanne B. Stewart-Merrill, Christine M. Lohse, Bradley C. Leibovich, Stephen A. Boorjian, R. Houston Thompson
Urology.2016; 96: 106. CrossRef - The Correlation of Tissue-Based Biomarkers in Primary and Metastatic Renal Cell Carcinoma Lesions: A Tissue Microarray Study
Sung Han Kim, Weon Seo Park, Eun Young Park, Boram Park, Jungnam Joo, Jae Young Joung, Ho Kyung Seo, Kang Hyun Lee, Jinsoo Chung
The Korean Journal of Urological Oncology.2016; 14(3): 152. CrossRef - Long-term follow-up and clinical course of a rare case of von Hippel-Lindau disease: A case report and review of the literature
YU ZOU, JINGJING XU, MINMING ZHANG
Oncology Letters.2016; 11(5): 3273. CrossRef - Genetic alterations in renal cell carcinoma with rhabdoid differentiation
Carmen M. Perrino, Vishwanathan Hucthagowder, Michael Evenson, Shashikant Kulkarni, Peter A. Humphrey
Human Pathology.2015; 46(1): 9. CrossRef - High expression of APRIL correlates with poor prognosis in clear cell renal cell carcinoma
Cheol Lee, Jeong-Whan Park, Ja Hee Suh, Kyung Chul Moon
Pathology - Research and Practice.2015; 211(11): 824. CrossRef - A Case of Cutaneous Metastasis from a Clear Cell Renal Cell Carcinoma with an Eosinophilic Cell Component to the Submandibular Region
Yusuke Amano, Sumie Ohni, Toshiyuki Ishige, Taku Homma, Tsutomu Yamada, Nobuyuki Nishimori, Norimichi Nemoto
Journal of Nihon University Medical Association.2015; 74(2): 73. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure


Fig. 1
Fig. 2
| Parameter | No. (%) (n = 81) | |
|---|---|---|
| Sex | Male | 64 (79.0) |
| Female | 17 (21.0) | |
| Fuhrman grade | I | 0 (0) |
| II | 19 (23.5) | |
| III | 48 (59.3) | |
| IV | 14 (17.3) | |
| Stage | I | 25 (30.9) |
| II | 12 (14.8) | |
| III | 16 (19.8) | |
| IV | 28 (34.6) |
| Metastatic site | No. (%) (n = 119) |
|---|---|
| Lung | 49 (41.2) |
| Bone | 26 (21.8) |
| Soft tissue | 14 (11.8) |
| Skin | 6 (5.0) |
| Pancreas | 5 (4.2) |
| Liver | 4 (3.4) |
| Adrenal gland | 4 (3.4) |
| Brain | 2 (1.7) |
| Palatine tonsil | 2 (1.7) |
| Gallbladder | 1 (0.8) |
| Nasal cavity | 1 (0.8) |
| Thyroid | 1 (0.8) |
| Parotid gland | 1 (0.8) |
| Colon | 1 (0.8) |
| Lymph node (mediastinum) | 1 (0.8) |
| Stomach | 1 (0.8) |
| Primary (n = 81) | No. | Metastatic (n = 119) | No. |
|---|---|---|---|
| CL | 37 | CL | 42 |
| CL+E | 4 | ||
| CL+R | 3 | ||
| R | 1 | ||
| UN | 2 | ||
| CL+E | 19 | CL | 12 |
| CL+E | 8 | ||
| E | 8 | ||
| R | 1 | ||
| CL+R | 15 | R | 10 |
| CL+R | 11 | ||
| CL+R+UN | 1 | ||
| CL+SA | 2 | CL | 1 |
| CL+SA | 3 | ||
| CL+E+R | 5 | CL | 2 |
| CL+E | 2 | ||
| CL+R | 2 | ||
| E+R | 1 | ||
| CL+R+SA | 2 | CL+R+SA | 2 |
| R+SA | 2 | ||
| CL+E+R+SA | 1 | E+R+SA | 1 |
| Histologic feature | Primary (n = 81) | Metastatic (n = 119) | p-value |
|---|---|---|---|
| Eosinophilic | 25 (30.9) | 24 (20.2) | .094 |
| Rhabdoid | 23 (28.4) | 35 (29.4) | .877 |
| Sarcomatoid | 5 (6.2) | 8 (6.7) | .707 |
| Unclassifiable | 0 (0) | 3 (2.5) | .158 |
| Case No. | Primary | 1st mets | 2nd mets | 3rd mets | 4th mets | 5th mets |
|---|---|---|---|---|---|---|
| 3 | CL+R | R (bone) | R (bone) | |||
| 14 | CL+R | R (skin) | R (skin) | |||
| 21 | CL+E | CL+E (soft ts) | CL+E (liver) | |||
| 28 | CL+R | CL+R (lung) | CL+R (soft ts) | |||
| 32 | CL+E | E (lung) | E (lung) | E (lung) | E (lung) | E (tonsil) |
| 33 | CL+SA | CL+SA (lung) | CL+SA (lung) | |||
| 38 | CL+R | R (bone) | R (bone) | |||
| 39 | CL+R | CL+R (lung) | CL+R (lung) | |||
| 41 | CL+R+SA | CL+R+SA (lung) | CL+R+SA (soft ts) | |||
| 47 | CL+R+SA | R+SA (soft ts) | R+SA (lung) | |||
| 48 | CL | CL+R (tonsil) | CL+R (skin) | |||
| 49 | CL+R | CL+R (lung) | CL+R (soft ts) | |||
| 79 | CL | CL+E (soft ts) | CL+E (liver) | CL+E (sto) | ||
| 12 | CL+E+R | CL+E (lung) | CL (lung) | CL (soft ts) | ||
| 16 | CL+E | CL (adrenal) | R (bone) | |||
| 69 | CL+R | CL+R (soft ts) | R (skin) | |||
| 73 | CL | CL+R (lung) | CL (lung) |
| Immunohistochemical marker | Positive cases/Total cases (%) |
p-value | |
|---|---|---|---|
| Primary | Metastatic | ||
| CD10 | 20/20 (100) | 45/46 (97.8) | .514 |
| RCC Ma | 3/5 (60) | 7/15 (46.7) | .628 |
| CK7 | 0/12 (0) | 1/18 (5.6) | .424 |
| CK20 | 0/7 (0) | 0/15 (0) | - |
| CK | 5/7 (71.4) | 9/20 (45.0) | .245 |
| EMA | 1/1 (100) | 11/13 (84.6) | - |
| VT | 7/12 (58.3) | 24/29 (82.8) | .158 |
| PAX2 | 4/4 (100) | 2/3 (66.7) | .423 |
CL, typical clear cell morphology; E, eosinophilic cytoplasm; R, rhabdoid feature; UN, unclassifiable; SA, sarcomatoid differentiation.
Values are presented as number (%).
Mets, metastasis; CL, typical clear cell morphology; R, rhabdoid feature; E, eosinophilic cytoplasm; soft ts, soft tissue; SA, sarcomatoid differentiation; sto, stomach.
Values are presented as number (%). RCC Ma, renal cell carcinoma marker; CK, cytokeratin; EMA, epithelial membrane antigen; VT, vimentin.

 E-submission
E-submission