Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 48(1); 2014 > Article
-
Brief Case Report
Crush Cytology of Microcystic Meningioma with Extensive Sclerosis - Jae Yeon Seok, Na Rae Kim, Hyun Yee Cho, Dong Hae Chung, Gi-Taek Yee1, Eung Yeop Kim2
-
Korean Journal of Pathology 2014;48(1):77-80.
DOI: https://doi.org/10.4132/KoreanJPathol.2014.48.1.77
Published online: February 25, 2014
Department of Pathology, Gachon University Gil Medical Center, Incheon, Korea.
1Department of Neurosurgery, Gachon University Gil Medical Center, Incheon, Korea.
2Department of Radiology, Gachon University Gil Medical Center, Incheon, Korea.
- Corresponding Author: Na Rae Kim, M.D. Department of Pathology, Gachon University Gil Medical Center, 21 Namdong-daero 774 beon-gil, Namdong-gu, Incheon 405-760, Korea. Tel: +82-32-460-3073, Fax: +82-32-460-2394, clara_nrk@gilhospital.com
• Received: May 14, 2013 • Revised: July 26, 2013 • Accepted: July 30, 2013
© 2014 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations
Citations to this article as recorded by 

- Cytologic features of meningioma: An analysis of common and uncommon subtypes and diagnostic difficulties during intraoperative procedures
Ana M. Rodríguez‐García, Isabel Esteban‐Rodríguez, José A. Jiménez‐Heffernan, Carmen Bárcena, Samuel López‐Muñoz, Pilar López‐Ferrer
Cytopathology.2024; 35(5): 581. CrossRef - Exploring the role of epidermal growth factor receptor variant III in meningeal tumors
Rashmi Rana, Vaishnavi Rathi, Kirti Chauhan, Kriti Jain, Satnam Singh Chhabra, Rajesh Acharya, Samir Kumar Kalra, Anshul Gupta, Sunila Jain, Nirmal Kumar Ganguly, Dharmendra Kumar Yadav, Timir Tripathi
PLOS ONE.2021; 16(9): e0255133. CrossRef - Intraoperative frozen cytology of intraosseous cystic meningioma in the sphenoid bone
Na Rae Kim, Gie-Taek Yie
Journal of Pathology and Translational Medicine.2020; 54(6): 508. CrossRef - Can amide proton transfer–weighted imaging differentiate tumor grade and predict Ki-67 proliferation status of meningioma?
Hao Yu, Xinrui Wen, Pingping Wu, Yueqin Chen, Tianyu Zou, Xianlong Wang, Shanshan Jiang, Jinyuan Zhou, Zhibo Wen
European Radiology.2019; 29(10): 5298. CrossRef - Intraoperative Frozen Cytology of Central Nervous System Neoplasms: An Ancillary Tool for Frozen Diagnosis
Myunghee Kang, Dong Hae Chung, Na Rae Kim, Hyun Yee Cho, Seung Yeon Ha, Sangho Lee, Jungsuk An, Jae Yeon Seok, Gie-Taek Yie, Chan Jong Yoo, Sang Gu Lee, Eun Young Kim, Woo Kyung Kim, Seong Son, Sun Jin Sym, Dong Bok Shin, Hee Young Hwang, Eung Yeop Kim, K
Journal of Pathology and Translational Medicine.2019; 53(2): 104. CrossRef - Crush Cytology of Secretory Meningioma: A Case Report
Na Rae Kim, Gie-Taek Yee, Hyun Yee Cho
Brain Tumor Research and Treatment.2015; 3(2): 147. CrossRef
Crush Cytology of Microcystic Meningioma with Extensive Sclerosis



Fig. 1 Enhanced T2-weighted images show a well-demarcated mass (arrow) of high signal intensity as well as low focal signal.
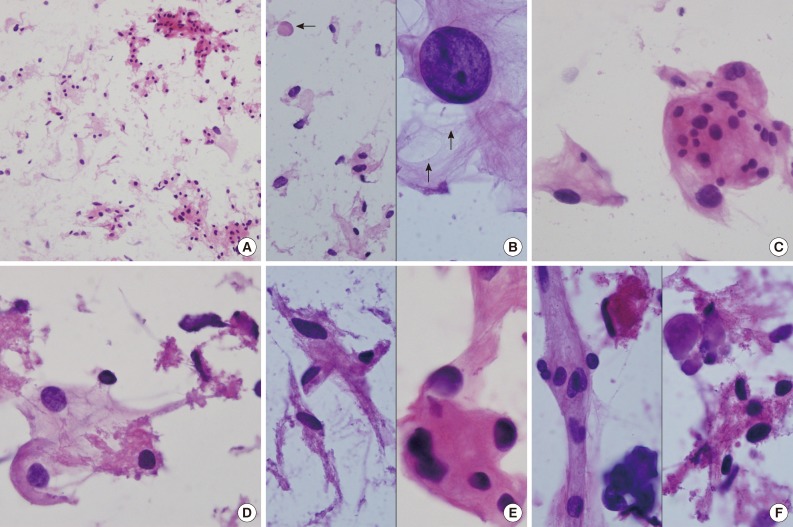
Fig. 2 (A) Crush cytology smears show sparse cellularity composed of scattered individual cells and a few clusters of eosinophilic cells in bluish mucinous materials in the background. (B) Left: Scattered polygonal cells have abundant vacuolated bluish cells. Note background bluish mucinous materials and solid hyaline materials (arrow). Right: Hyperchromatic round nuclei and lacy cytoplasm with microcysts (arrows). (C) Meningothelial whorls of eosinophilic cells have indistinct cell borders and occasional nuclear hyperchromasia. (D) The vacuolated cytoplasm also shows collagenous hyalinized cytoplasm. (E) Left: Collagen-deposited cells show low nuclear/cytoplasmic ratio. Right: Note refractile and eosinophilic hyaline cytoplasm with feathering off. (F) Calcified materials and collagens are scattered in background.
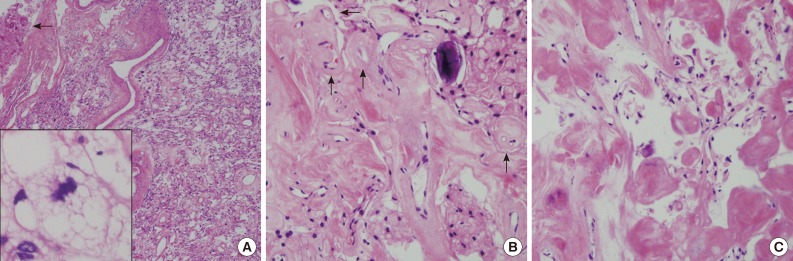
Fig. 3 (A) The resected mass is composed of mainly sclerosed and fibrotic collagenous deposits; however, the characteristic portion of microcystic meningioma is also found. Arrow indicates adhered brain parenchyma without tongue-like tumor invasion. Inset indicates stellate, spider-like multivacuolated cells in the microcystic area. (B) In the fibrotic portion, the residual meningioma cells are transformed to massive sclerosis. Arrows indicate hyalinized vessels. (C) High magnification of the sclerotic portion shows some spindle-shaped fibroblast-like cells in abundant eosinophilic, collagenous deposits.
Fig. 1
Fig. 2
Fig. 3
Crush Cytology of Microcystic Meningioma with Extensive Sclerosis

 E-submission
E-submission

 PubReader
PubReader Cite this Article
Cite this Article