Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 48(2); 2014 > Article
-
Original Article
Characteristics of Cutaneous Lymphomas in Korea According to the New WHO-EORTC Classification: Report of a Nationwide Study - Jae Ho Han, Young-Hyeh Ko1, Yun Kyung Kang2, Wan-Seop Kim3, Yoon Jung Kim4, Insun Kim5, Hyun-Jung Kim6, Soo Kee Min7, Chan-Kum Park8, Chan-Sik Park9, Bong-Kyung Shin10, Woo Ick Yang11, Young-Ha Oh12, Jong Sil Lee13, Juhie Lee14, Tae Hui Lee15, Hyekyung Lee16, Ho Jung Lee17, Yoon Kyung Jeon18, Hee Jeong Cha19, Yoo-Duk Choi20, Chul Woo Kim18, Hematopathology Study Group of the Korean Society of Pathologists
-
Korean Journal of Pathology 2014;48(2):126-132.
DOI: https://doi.org/10.4132/KoreanJPathol.2014.48.2.126
Published online: April 28, 2014
Department of Pathology, Ajou University School of Medicine, Suwon, Korea.
1Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
2Department of Pathology, Inje University Seoul Paik Hospital, Seoul, Korea.
3Department of Pathology, Konkuk University School of Medicine, Seoul, Korea.
4Department of Pathology, Veterans Health Service Medical Center, Seoul, Korea.
5Department of Pathology, Korea University College of Medicine, Seoul, Korea.
6Department of Pathology, Inje University Sanggye Paik Hospital, Seoul, Korea.
7Department of Pathology, Hallym University Sacred Heart Hospital, Anyang, Korea.
8Department of Pathology, Hanyang University College of Medicine, Seoul, Korea.
9Department of Pathology, Asan Medical Center, Seoul, Korea.
10Department of Pathology, Korea University Guro Hospital, Seoul, Korea.
11Department of Pathology, Yonsei University College of Medicine, Seoul, Korea.
12Department of Pathology, Hanyang University Guri Hospital, Guri, Korea.
13Department of Pathology, Gyeongsang National University School of Medicine, Jinju, Korea.
14Department of Pathology, Kyung Hee University School of Medicine, Seoul, Korea.
15Department of Pathology, Dankook University College of Medicine, Cheonan, Korea.
16Department of Pathology, Eulji University Hospital, Daejeon, Korea.
17Department of Pathology, Eulji General Hospital, Seoul, Korea.
18Department of Pathology, Seoul National University, Seoul, Korea.
19Department of Pathology, Ulsan University College of Medicine, Ulsan, Korea.
20Department of Pathology, Chonnam National University Medical School, Gwangju, Korea.
- Corresponding Author: Chul Woo Kim, M.D. Department of Pathology, Seoul National University, 101 Daehak-ro, Jongno-gu, Seoul 110-744, Korea. Tel: +82-2-740-8267, Fax: +82-2-743-5530, cwkim@snu.ac.kr
© 2014 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations

- Varied presentations of primary cutaneous lymphoma: A case series from a tertiary care center in South India
Baby Shana, Betsy Ambooken, Sunitha Balakrishnan, Asokan Neelakandan, Kidangazhiyathmana Ajithkumar
Indian Journal of Cancer.2024; 61(1): 172. CrossRef - A retrospective study of prognostic factors and treatment outcome in advanced-stage Mycosis Fungoides and Sezary Syndrome
Zhuo-fan Xu, Hongyun Chen, Yuehua Liu, Wei Zhang, Hongzhong Jin, Jie Liu
Hematology.2024;[Epub] CrossRef - Prevalence, clinical features, and survival outcome trends of 627 patients with primary cutaneous lymphoma over 29 years: a retrospective review from single tertiary center in Korea
Ik Jun Moon, Chong Hyun Won, Sung Eun Chang, Chan-Sik Park, Dok-Hyun Yoon, Si Yeol Song, Mi Woo Lee, Woo Jin Lee
Scientific Reports.2024;[Epub] CrossRef - The First Case of Acute Myeloid Leukemia With t(10;11)(p13;q21);PICALM-MLLT10 Rearrangement Presenting With Extensive Skin Involvement
Min-Seung Park, Hyun-Young Kim, Jae Joon Lee, Duck Cho, Chul Won Jung, Hee-Jin Kim, Sun-Hee Kim
Annals of Laboratory Medicine.2023; 43(3): 310. CrossRef - Recent advances on cutaneous lymphoma epidemiology
G. Dobos, M. Miladi, L. Michel, C. Ram-Wolff, M. Battistella, M. Bagot, A. de Masson
La Presse Médicale.2022; 51(1): 104108. CrossRef - Specific cutaneous infiltrates in patients with haematological neoplasms: a retrospective study with 49 patients
Rebeca Calado, Maria Relvas, Francisca Morgado, José Carlos Cardoso, Oscar Tellechea
Australasian Journal of Dermatology.2021;[Epub] CrossRef - Epidemiology of Cutaneous T-Cell Lymphomas: A Systematic Review and Meta-Analysis of 16,953 Patients
Gabor Dobos, Anne Pohrt, Caroline Ram-Wolff, Céleste Lebbé, Jean-David Bouaziz, Maxime Battistella, Martine Bagot, Adèle de Masson
Cancers.2020; 12(10): 2921. CrossRef - Primary cutaneous lymphoma in Argentina: a report of a nationwide study of 416 patients
Alejandra Abeldaño, Paula Enz, Matias Maskin, Andrea B. Cervini, Natallia Torres, Ana C. Acosta, Marina Narbaitz, Silvia Vanzulli, Mirta Orentrajch, Marta A. Villareal, Maria L. Garcia Pazos, Mariana Arias, Evelyn A. Zambrano Franco, Maria I. Fontana, Rob
International Journal of Dermatology.2019; 58(4): 449. CrossRef - Post-thymic CD4 positive cytotoxic T cell infiltrates of the skin: A clinical and histomorphologic spectrum of the unique CD4 positive T cell of immunosenescence
Cynthia M. Magro, Luke C. Olson, Shabnam Momtahen
Annals of Diagnostic Pathology.2019; 38: 99. CrossRef - Cutaneous lymphomas in Taiwan: A review of 118 cases from a medical center in southern Taiwan
Chaw-Ning Lee, Chao-Kai Hsu, Kung-Chao Chang, Cheng-Lin Wu, Tsai-Yun Chen, Julia Yu-Yun Lee
Dermatologica Sinica.2018; 36(1): 16. CrossRef - Imaging analysis of superficial soft tissue lymphomas
In Sook Lee, You Seon Song, Seung Hyun Lee, Young Jin Choi, Sung Moon Lee
Clinical Imaging.2018; 49: 111. CrossRef - Epidemiologic, clinical and demographic features of primary cutaneous lymphomas in Castilla‐La Mancha, Spain: are we different?
C. Ramos‐Rodríguez, M. García‐Rojo, G. Romero‐Aguilera, M. García‐Arpa, L. González‐López, M.P. Sánchez‐Caminero, J. González‐García, M. Delgado‐Portela, M.P. Cortina‐De La Calle, M.F. Relea‐Calatayud, F. Martín‐Dávila, R. López‐Pérez, M. Ramos‐Rodríguez
Journal of the European Academy of Dermatology and Venereology.2018;[Epub] CrossRef - Nasal-type NK/T-cell lymphomas are more frequently T rather than NK lineage based on T-cell receptor gene, RNA, and protein studies: lineage does not predict clinical behavior
Mineui Hong, Taehee Lee, So Young Kang, Suk-Jin Kim, Wonseog Kim, Young-Hyeh Ko
Modern Pathology.2016; 29(5): 430. CrossRef - Cutaneous lymphoma: Kids are not just little people
Katalin Ferenczi, Hanspaul S. Makkar
Clinics in Dermatology.2016; 34(6): 749. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure


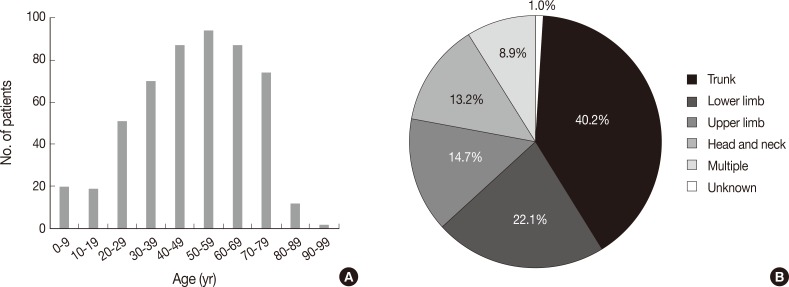
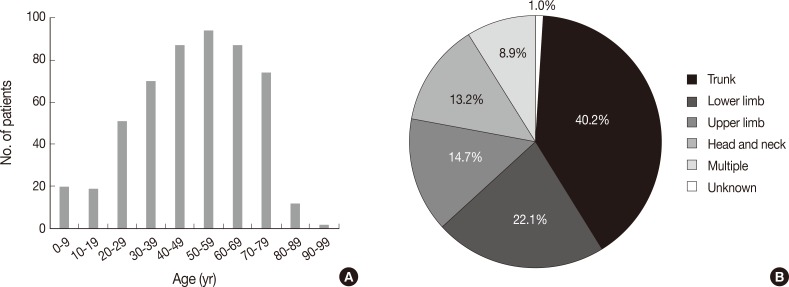
Fig. 1
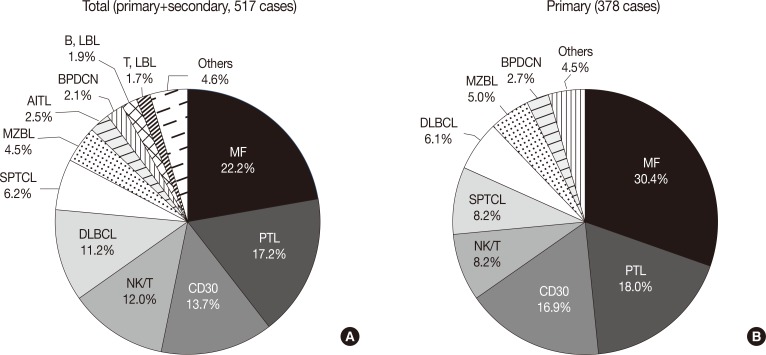
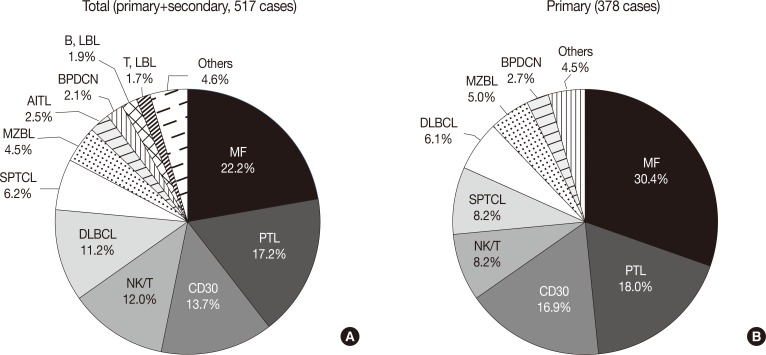
Fig. 2
| Total | Primary | Secondary | |
|---|---|---|---|
| Mature T-cell lymphoma | |||
| Mycosis fungoides | 110 (21.3) | 110 (29.1) | - |
| Pagetoid reticulosis | 1 (0.2) | 1 (0.3) | - |
| Follicular, syringotropic, granulomatous variants | 3 (0.6) | 3 (0.8) | - |
| Granulomatous slack skin | 1 (0.2) | 1 (0.3) | - |
| Sezary syndrome | 1 (0.2) | 1 (0.3) | - |
| CD30+ T-cell lymphoproliferative disorders | 3 (0.6) | 1 (0.3) | 2 (1.5) |
| Lymphomatoid papulosis | 32 (6.2) | 32 (8.5) | - |
| Anaplastic large cell lymphoma | 36 (7.0) | 31 (8.2) | 5 (3.7) |
| Subcutaneous panniculitis-like T-cell lymphoma | 32 (6.2) | 31 (8.2) | 1 (0.8) |
| Peripheral T-cell lymphoma, unspecified | 71 (13.7) | 51 (13.5) | 19 (14.2) |
| Aggressive epidermotropic CD8-positive cytotoxic T-cell lymphoma | 4 (0.8) | 4 (1.1) | - |
| Gamma/delta-positive T-cell lymphoma | 9 (1.7) | 8 (2.1) | 1 (0.8) |
| Small/medium CD4+ T-cell lymphoma | 5 (1.0) | 5 (1.3) | - |
| Extranodal NK/T cell lymphoma | 58 (11.2) | 27 (7.1) | 30 (22.4) |
| Hydroa vacciniforme-like lymphoma | 4 (0.8) | 4 (1.1) | - |
| Adult T-cell leukemia/lymphoma | 1 (0.2) | - | - |
| Angioimmunoblastic T-cell lymphoma | 13 (2.5) | 1 (0.3) | 12 (9.0) |
| Mature B-cell lymphoma | |||
| Marginal zone B-cell lymphoma | 23 (4.5) | 19 (5.0) | 4 (3.0) |
| Follicle centre lymphoma | 4 (0.8) | 4 (1.1) | - |
| Diffuse large B-cell lymphoma | 43 (8.3) | 17 (4.5) | 26 (19.4) |
| Diffuse large B-cell lylmphoma, leg type | 9 (1.7) | 6 (1.6) | 3 (2.2) |
| Diffuse large B-cell lymphoma, other | 5 (1.0) | - | 4 (3.0) |
| Plasmablastic lymphoma | 1 (0.2) | - | 1 (0.8) |
| Intravascular large B-cell lymphoma | 4 (0.8) | 2 (0.5) | 2 (1.5) |
| Lymphomatoid granulomatosis | 1 (0.2) | 1 (0.3) | - |
| Chronic lymphocytic leukemia | 1 (0.2) | - | 1 (0.8) |
| Burkitt lymphoma | 2 (0.4) | - | 2 (1.5) |
| Immature hematopoietic malignancies | |||
| Blastic plasmacytoid dendritic cell neoplasm | 11 (2.1) | 10 (2.7) | 1 (0.8) |
| T-lymphoblastic lymphoma/leukemia | 9 (1.7) | 2 (0.5) | 7 (5.2) |
| B-lymphoblastic lymphoma/leukemia | 10 (1.9) | 3 (0.8) | 7 (5.2) |
| Hodgkin’s lymphoma | 1 (0.2) | - | 1 (0.8) |
| Others | 9 (1.7) | 3 (0.8) | 6 (4.5) |
| No. of patients (%) | Sex (M/F) | Age distribution (rate, %) |
||||
|---|---|---|---|---|---|---|
| 0-20 yr | 21-40 yr | 41-60 yr | >60 yr | |||
| MF | 115 (22.2) | 1.1 | 3.5 | 36.5 | 41.7 | 18.3 |
| CD30 | 71 (13.7) | 1.3 | 21.1 | 21.1 | 21.1 | 21.1 |
| SPTCL | 32 (6.2) | 0.5 | 15.6 | 50.0 | 25.0 | 9.4 |
| PTL | 89 (17.2) | 1.1 | 5.6 | 21.3 | 38.2 | 34.8 |
| NK/T | 62 (12.0) | 1.5 | 11.3 | 16.1 | 29.0 | 43.5 |
| AITL | 13 (2.5) | 5.5 | 0.0 | 0.0 | 23.1 | 76.9 |
| MZBL | 23 (4.5) | 1.9 | 0.0 | 21.7 | 60.9 | 17.4 |
| DLBCL | 58 (11.2) | 1.9 | 0.0 | 8.6 | 29.3 | 62.1 |
| BPDCN | 11 (2.1) | 10.0 | 9.1 | 36.4 | 9.1 | 45.5 |
| T, LBL | 9 (1.7) | 8.0 | 11.1 | 44.4 | 44.4 | 0.0 |
| B, LBL | 10 (1.9) | 0.7 | 80.0 | 0.0 | 10.0 | 10.0 |
| Others | 24 (4.7) | 2.0 | 4.2 | 0.0 | 33.3 | 62.5 |
| No. of patients (%) | Anatomic location (rate, %) |
|||||
|---|---|---|---|---|---|---|
| Head and neck | Trunk | Upper limb | Lower limb | Multiple | ||
| MF | 115 (22.2) | 4.4 | 53.5 | 7.0 | 16.7 | 18.4 |
| CD30 | 71 (13.7) | 8.6 | 28.6 | 28.6 | 27.1 | 7.1 |
| SPTCL | 32 (6.2) | 3.1 | 53.1 | 12.5 | 12.5 | 18.8 |
| PTL | 89 (17.2) | 15.9 | 37.5 | 13.6 | 26.1 | 6.8 |
| NK/T | 62 (12.0) | 6.5 | 30.6 | 17.7 | 38.7 | 6.5 |
| AITL | 13 (2.5) | 30.8 | 46.2 | 23.1 | 0.0 | 0.0 |
| MZBL | 23 (4.5) | 52.2 | 39.1 | 8.7 | 0.0 | 0.0 |
| DLBCL | 58 (11.2) | 12.5 | 42.9 | 21.4 | 21.4 | 1.8 |
| BPDCN | 11 (2.1) | 27.3 | 36.4 | 9.1 | 18.2 | 9.1 |
| T, LBL | 9 (1.7) | 33.3 | 33.3 | 0.0 | 22.2 | 11.1 |
| B, LBL | 10 (1.9) | 60.0 | 10.0 | 10.0 | 20.0 | 0.0 |
| Others | 24 (4.7) | 12.5 | 45.8 | 8.3 | 29.2 | 4.2 |
| Western (%)5 | Present (%) | |
|---|---|---|
| Mature T-cell lymphoma | 71.3 | 82.3 |
| MF | 38.3 | 30.4 |
| SS | 0.8 | 0.3 |
| CD30 | 10.2 | 16.9 |
| SPTCL | 0.6 | 8.2 |
| PTL | 20.8 | 18.0 |
| NK/T | 0.3 | 8.2 |
| AITL | 0.2 | 0.3 |
| Mature B-cell lymphoma | 28.5 | 13.0 |
| MZBL | 7.1 | 5.0 |
| FL | 8.5 | 1.1 |
| DLBCL | 11.4 | 6.1 |
| Immature hematologic malignancies | 0.3 | 4.0 |
| BPDCN | 0.2 | 2.7 |
| T, LBL | 0.0 | 0.5 |
| B, LBL | 0.1 | 0.8 |
| Others | 1.5 | 0.8 |
Values are presented as number (%). WHO, World Health Organization; EORTC, European Organization for the Research and Treatment of Cancer; NK, natural killer.
M, male; F, female; MF, mycosis fungoides; CD30, CD30+ T-cell lymphoproliferative disorder; SPTCL, subcutaneous panniculitis-like T cell lymphoma; PTL, peripheral T cell lymphoma; NK/T, NK/T cell lymphoma, nasal type; AITL, angioimmunoblastic T cell lymphoma; MZBL, marginal zone B-cell lymphoma; DLBCL, diffuse large B-cell lymphoma; BPDCN, blastic plasmacytoid dendritic cell neoplasm; LBL, lymphoblastic lymphoma.
MF, mycosis fungoides; CD30, CD30+ T-cell lymphoproliferative disorder; SPTCL, subcutaneous panniculitis-like T cell lymphoma; PTL, peripheral T cell lymphoma; NK/T, NK/T cell lymphoma, nasal type; AITL, angioimmunoblastic T cell lymphoma; MZBL, marginal zone B-cell lymphoma; DLBCL, diffuse large B-cell lymphoma; BPDCN, blastic plasmacytoid dendritic cell neoplasm; LBL, lymphoblastic lymphoma.
MF, mycosis fungoides; SS, Sezary syndrome; CD30, CD30+ T-cell lymphoproliferative disorder; SPTCL, subcutaneous panniculitis-like T cell lymphoma; PTL, peripheral T cell lymphoma; NK/T, NK/T cell lymphoma, nasal type; AITL, angioimmunoblastic T cell lymphoma; MZBL, marginal zone B-cell lymphoma; FL, follicular lymphoma; DLBCL, diffuse large B-cell lymphoma; BPDCN, blastic plasmacytoid dendritic cell neoplasm; LBL, lymphoblastic lymphoma.

 E-submission
E-submission