Search
- Page Path
- HOME > Search
- Histopathologic classification and immunohistochemical features of papillary renal neoplasm with potential therapeutic targets
- Jeong Hwan Park, Su-Jin Shin, Hyun-Jung Kim, Sohee Oh, Yong Mee Cho
- J Pathol Transl Med. 2024;58(6):321-330. Published online September 12, 2024
- DOI: https://doi.org/10.4132/jptm.2024.07.31
- 6,278 View
- 423 Download
- 1 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Papillary renal cell carcinoma (pRCC) is the second most common histological subtype of renal cell carcinoma and is considered a morphologically and molecularly heterogeneous tumor. Accurate classification and assessment of the immunohistochemical features of possible therapeutic targets are needed for precise patient care. We aimed to evaluate immunohistochemical features and possible therapeutic targets of papillary renal neoplasms
Methods
We collected 140 papillary renal neoplasms from three different hospitals and conducted immunohistochemical studies on tissue microarray slides. We performed succinate dehydrogenase B, fumarate hydratase, and transcription factor E3 immunohistochemical studies for differential diagnosis and re-classified five cases (3.6%) of papillary renal neoplasms. In addition, we conducted c-MET, p16, c-Myc, Ki-67, p53, and stimulator of interferon genes (STING) immunohistochemical studies to evaluate their pathogenesis and value for therapeutic targets.
Results
We found that c-MET expression was more common in pRCC (classic) (p = .021) among papillary renal neoplasms and Ki-67 proliferation index was higher in pRCC (not otherwise specified, NOS) compared to that of pRCC (classic) and papillary neoplasm with reverse polarity (marginal significance, p = .080). Small subsets of cases with p16 block positivity (4.5%) (pRCC [NOS] only) and c-Myc expression (7.1%) (pRCC [classic] only) were found. Also, there were some cases showing STING expression and those cases were associated with increased Ki-67 proliferation index (marginal significance, p = .063).
Conclusions
Our findings suggested that there are subsets of pRCC with c-MET, p16, c-MYC, and STING expression and those cases could be potential candidates for targeted therapy. -
Citations
Citations to this article as recorded by- Tissue-Based Biomarkers Important for Prognostication and Management of Genitourinary Tumors, Including Surrogate Markers of Genomic Alterations
Leonie Beauchamp, Shreeya Indulkar, Eric Erak, Mohammad Salimian, Andres Matoso
Surgical Pathology Clinics.2025; 18(1): 175. CrossRef - Papillary renal neoplasm with reverse polarity: a case report and literature review
Diego Gonzalez, Kris Kokoneshi, Sam Kwon, Ryan Thomas Mathews, Ryan Michael Antar, Maher Ali, Abiye Kassa, Michael Whalen
Frontiers in Oncology.2025;[Epub] CrossRef
- Tissue-Based Biomarkers Important for Prognostication and Management of Genitourinary Tumors, Including Surrogate Markers of Genomic Alterations
- Loss of aquaporin-1 expression is associated with worse clinical outcomes in clear cell renal cell carcinoma: an immunohistochemical study
- Seokhyeon Lee, Bohyun Kim, Minsun Jung, Kyung Chul Moon
- J Pathol Transl Med. 2023;57(4):232-237. Published online July 11, 2023
- DOI: https://doi.org/10.4132/jptm.2023.06.17
- 4,711 View
- 171 Download
- 2 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Aquaporin (AQP) expression has been investigated in various malignant neoplasms, and the overexpression of AQP is related to poor prognosis in some malignancies. However, the expression of AQP protein in clear cell renal cell carcinoma (ccRCC) has not been extensively investigated by immunohistochemistry with large sample size.
Methods
We evaluated the AQP expression in 827 ccRCC with immunohistochemical staining in tissue microarray blocks and classified the cases into two categories, high and low expression.
Results
High expression of aquaporin-1 (AQP1) was found in 320 cases (38.7%), but aquaporin-3 was not expressed in ccRCC. High AQP1 expression was significantly related to younger age, low TNM stage, low World Health Organization/International Society of Urologic Pathology nuclear grade, and absence of distant metastasis. Furthermore, high AQP1 expression was also significantly associated with longer overall survival (OS; p<.001) and progression-specific survival (PFS; p<.001) and was an independent predictor of OS and PFS in ccRCC.
Conclusions
Our study revealed the prognostic significance of AQP1 protein expression in ccRCC. These findings could be applied to predict the prognosis of ccRCC. -
Citations
Citations to this article as recorded by- Loss of Aquaporin-1 in Tumor Cells Fosters Intrahepatic Cholangiocarcinoma Progression
César I. Gaspari, Carine Beaupere, Seth Richard, Estanislao Peixoto, Bouchra Lekbaby, Mirko Minini, Branko Dubravcic, Javier Vaquero, Marie Vallette, Ander Arbelaiz, Marion Janona, Corentin Louis, Pauline Le Gall, Cédric Coulouarn, Julieta Marrone, Juan E
The American Journal of Pathology.2026; 196(2): 428. CrossRef - Construction and validation of renal cell carcinoma tumor cell differentiation-related prognostic classification (RCC-TCDC): an integrated bioinformatic analysis and clinical study
Yifan Liu, Keqin Dong, Yuntao Yao, Bingnan Lu, Lei Wang, Guo Ji, Haoyu Zhang, Zihui Zhao, Xinyue Yang, Runzhi Huang, Wang Zhou, Xiuwu Pan, Xingang Cui
Annals of Medicine.2025;[Epub] CrossRef - Prognostic Assessment of Aquaporins in Pancreatic Adenocarcinoma: An In Silico Analysis
Vignesh Krishnasamy, Lalhmingliana, Nachimuthu Senthil Kumar
Current Biotechnology.2025; 14(2): 130. CrossRef - Targeting PLOD2 induces epithelioid differentiation and improves therapeutic response in sarcomatoid renal cell carcinoma
Xiangyu Chen, Dongkui Xu, Yu Ji, Xichen Dong, Xiaomei Dong, Zihan Li, Jingyu Tan, Qianqian Sun, Huixian Xin, Ziwei Liu, Qing Deng, Tao Wen, Yanjun Jia, Xuhui Zhu, Jian Liu
Journal of Advanced Research.2025;[Epub] CrossRef - Serum Exosomal MiR-874 as a Potential Biomarker for Nonsmall Cell Lung Cancer Diagnosis and Prognosis
Amal F. Gharib, Saad S. Al-Shehri, Abdulraheem Almalki, Ayman Alhazmi, Mamdouh Allahyani, Ahmed Alghamdi, Amani A. Alrehaili, Maha M. Bakhuraysah, Althobaiti Naif Saad M., Weal H. Elsawy
Indian Journal of Medical and Paediatric Oncology.2024;[Epub] CrossRef
- Loss of Aquaporin-1 in Tumor Cells Fosters Intrahepatic Cholangiocarcinoma Progression
- Loss of Nuclear BAP1 Expression Is Associated with High WHO/ISUP Grade in Clear Cell Renal Cell Carcinoma
- Young Chan Wi, Ahrim Moon, Min Jung Jung, Yeseul Kim, Seong Sik Bang, Kiseok Jang, Seung Sam Paik, Su-Jin Shin
- J Pathol Transl Med. 2018;52(6):378-385. Published online October 1, 2018
- DOI: https://doi.org/10.4132/jptm.2018.09.21
- 10,802 View
- 228 Download
- 14 Web of Science
- 14 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
BRCA1-associated protein 1 (BAP1) mutations are frequently reported in clear cell renal cell carcinoma (ccRCC); however, very few studies have evaluated the role of these mutations in other renal cell carcinoma (RCC) subtypes. Therefore, we analyzed BAP1 protein expression using immunohistochemistry in several RCC subtypes and assessed its relationship with clinicopathological characteristics of patients.
Methods
BAP1 expression was immunohistochemically evaluated in tissue microarray blocks constructed from 371 samples of RCC collected from two medical institutions. BAP1 expression was evaluated based on the extent of nuclear staining in tumor cells, and no expression or expression in < 10% of tumor cells was defined as negative.
Results
Loss of BAP1 expression was observed in ccRCC (56/300, 18.7%), chromophobe RCC (6/26, 23.1%), and clear cell papillary RCC (1/4, 25%), while we failed to detect BAP1 expression loss in papillary RCC, acquired cystic disease-associated RCC, or collecting duct carcinoma. In ccRCC, loss of BAP1 expression was significantly associated with high World Health Organization (WHO)/International Society of Urological Pathology (ISUP) grade (p = .002); however, no significant correlation was observed between loss of BAP1 expression and survival in ccRCC. Loss of BAP1 expression showed no association with prognostic factors in chromophobe RCC.
Conclusions
Loss of BAP1 nuclear expression was observed in both ccRCC and chromophobe RCC. In addition, BAP1 expression loss was associated with poor prognostic factors such as high WHO/ISUP grade in ccRCC. -
Citations
Citations to this article as recorded by- The Role of Homologous Recombination Deficiency (HRD) in Renal Cell Carcinoma (RCC): Biology, Biomarkers, and Therapeutic Opportunities
Alberto Bongiovanni, Pierfranco Conte, Vincenza Conteduca, Matteo Landriscina, Giuseppe Di Lorenzo, Francesco Cognetti
Current Oncology.2025; 32(12): 690. CrossRef - Clinical and Genomic Features of Patients with Renal Cell Carcinoma and Advanced Chronic Kidney Disease: Analysis of a Multi-Institutional Database
Corbin J. Eule, Junxiao Hu, Dale Hedges, Alkesh Jani, Thomas Pshak, Brandon J. Manley, Alejandro Sanchez, Robert Dreicer, Zin W. Myint, Yousef Zakharia, Elaine T. Lam
Cancers.2024; 16(10): 1920. CrossRef - Immune regulation and prognosis indicating ability of a newly constructed multi-genes containing signature in clear cell renal cell carcinoma
Ziwei Gui, Juan Du, Nan Wu, Ningning Shen, Zhiqing Yang, Huijun Yang, Xuzhi Wang, Na Zhao, Zixin Zeng, Rong Wei, Wenxia Ma, Chen Wang
BMC Cancer.2023;[Epub] CrossRef - Radiogenomic Associations Clear Cell Renal Cell Carcinoma: An Exploratory Study
Derek H Liu, Komal A Dani, Sharath S Reddy, Xiaomeng Lei, Natalie L Demirjian, Darryl H Hwang, Bino A Varghese, Suhn Kyong Rhie, Felix Y. Yap, David I. Quinn, Imran Siddiqi, Manju Aron, Ulka Vaishampayan, Haris Zahoor, Steven Y Cen, Inderbir S Gill, Vinay
Oncology.2023; 101(6): 375. CrossRef - Immunohistochemistry for the diagnosis of renal epithelial neoplasms
Mahmut Akgul, Sean R Williamson
Seminars in Diagnostic Pathology.2022; 39(1): 1. CrossRef - BRCA1-Associated Protein 1 (BAP-1) as a Prognostic and Predictive Biomarker in Clear Cell Renal Cell Carcinoma: A Systematic Review
Shuchi Gulati, Melissa Previtera, Primo N. Lara
Kidney Cancer.2022; 6(1): 23. CrossRef - Renal Cell Carcinoma in End-Stage Renal Disease: A Review and Update
Ziad M. El-Zaatari, Luan D. Truong
Biomedicines.2022; 10(3): 657. CrossRef - CD117, BAP1, MTAP, and TdT Is a Useful Immunohistochemical Panel to Distinguish Thymoma from Thymic Carcinoma
Mounika Angirekula, Sindy Y Chang, Sarah M. Jenkins, Patricia T. Greipp, William R. Sukov, Randolph S. Marks, Kenneth R. Olivier, Stephen D. Cassivi, Anja C Roden
Cancers.2022; 14(9): 2299. CrossRef - BAP1 in cancer: epigenetic stability and genome integrity
Sabrina Caporali, Alessio Butera, Ivano Amelio
Discover Oncology.2022;[Epub] CrossRef - Bioinformatic analysis identifying FGF1 gene as a new prognostic indicator in clear cell Renal Cell Carcinoma
Xiaoqin Zhang, Ziyue Wang, Zixin Zeng, Ningning Shen, Bin Wang, Yaping Zhang, Honghong Shen, Wei Lu, Rong Wei, Wenxia Ma, Chen Wang
Cancer Cell International.2021;[Epub] CrossRef - Identification of Four Pathological Stage‐Relevant Genes in Association with Progression and Prognosis in Clear Cell Renal Cell Carcinoma by Integrated Bioinformatics Analysis
Dengyong Xu, Yuzi Xu, Yiming Lv, Fei Wu, Yunlong Liu, Ming Zhu, Dake Chen, Bingjun Bai, Rui Liu
BioMed Research International.2020;[Epub] CrossRef - Functional characterisation guides classification of novel BAP1 germline variants
Jing Han Hong, Siao Ting Chong, Po-Hsien Lee, Jing Tan, Hong Lee Heng, Nur Diana Binte Ishak, Sock Hoai Chan, Bin Tean Teh, Joanne Ngeow
npj Genomic Medicine.2020;[Epub] CrossRef - Tissue-Based Immunohistochemical Markers for Diagnosis and Classification of Renal Cell Carcinoma
Liang G Qu, Vaisnavi Thirugnanasundralingam, Damien Bolton, Antonio Finelli, Nathan Lawrentschuk
Société Internationale d’Urologie Journal.2020; 1(1): 68. CrossRef - Radiogenomics: bridging imaging and genomics
Zuhir Bodalal, Stefano Trebeschi, Thi Dan Linh Nguyen-Kim, Winnie Schats, Regina Beets-Tan
Abdominal Radiology.2019; 44(6): 1960. CrossRef
- The Role of Homologous Recombination Deficiency (HRD) in Renal Cell Carcinoma (RCC): Biology, Biomarkers, and Therapeutic Opportunities
- An Intrarenal Adrenocortical Carcinoma Arising in an Adrenal Rest
- Ji Hee Lee, Young Deuk Choi, Nam Hoon Cho
- J Pathol Transl Med. 2018;52(6):416-419. Published online October 1, 2018
- DOI: https://doi.org/10.4132/jptm.2018.07.20
- 7,239 View
- 97 Download
- 3 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF - We describe a case of a 61-year-old Korean man who was diagnosed with renal cell carcinoma that was discovered on abdominopelvic computed tomography obtained after the patient complained of back pain. A radical nephrectomy was performed, and the surgical specimen showed a relatively well-circumscribed and yellowish lobulated hard mass. Microscopically, the tumor showed sheets and nests of hypercellular pleomorphic cells with thick fibrous septation, frequent mitoses, and areas of adrenal cortical-like tissue. Immunohistochemical staining revealed that the tumor cells were positive for inhibin-α, vimentin, synaptophysin, and melan A. It also revealed that the tumor cells were negative for pan-cytokeratin, epithelial membrane antigen, paired box 8, α-methylacyl-coenzyme A racemase, CD10, cytokeratin 7, carbonic anhydrase 9, c-Kit, renal cell carcinoma, transcription factor E3, human melanoma black 45, desmin, smooth muscle actin, S-100, chromogranin A, CD34, anaplastic lymphoma kinase, and integrase interactor 1. Based on these histopathological and immunohistochemical findings, we diagnosed the tumor as intrarenal adrenocortical carcinoma arising in an adrenal rest. Several cases of intrarenal adrenocortical carcinoma have been reported, although they are very rare. Due to its poor prognosis and common recurrence or metastasis, clinicians and pathologists must be aware of this entity.
-
Citations
Citations to this article as recorded by- Non-functional Adrenocortical Carcinoma in the Wall of the Small Bowel
Shu-Juan Lin, Yan Gao, Chun-Juan Sun
Current Medical Imaging Reviews.2023;[Epub] CrossRef - Ectopic adrenal tissue in the kidney: A systematic review
Davide De Marchi, Alessandro Tafuri, Guglielmo Mantica, Aliasger Shakir, Federico Scarfò, Giovanni Passaretti, Salvatore Smelzo, Silvia Proietti, Lorenzo Rigatti, Roberta Luciano, Alessandro Antonelli, Vincenzo Pagliarulo, Rosario Leonardi, Gu
Archivio Italiano di Urologia e Andrologia.2021; 93(4): 481. CrossRef - Extra-adrenal, non-functional adrenocortical carcinoma presenting with acute abdomen: a case report
Alireza Mirsharifi, Mohammad Vasei, Ehsan Sadeghian, Ali Ghorbani-Abdehgah, Sara Naybandi Atashi
Journal of Medical Case Reports.2020;[Epub] CrossRef - Testicular Adrenal Rest Tumors: Current Insights on Prevalence, Characteristics, Origin, and Treatment
Manon Engels, Paul N Span, Antonius E van Herwaarden, Fred C G J Sweep, Nike M M L Stikkelbroeck, Hedi L Claahsen-van der Grinten
Endocrine Reviews.2019; 40(4): 973. CrossRef
- Non-functional Adrenocortical Carcinoma in the Wall of the Small Bowel
- Significance of Intratumoral Fibrosis in Clear Cell Renal Cell Carcinoma
- Jae Won Joung, Hoon Kyu Oh, Sun Jae Lee, Young Ah Kim, Hyun Jin Jung
- J Pathol Transl Med. 2018;52(5):323-330. Published online August 19, 2018
- DOI: https://doi.org/10.4132/jptm.2018.07.21
- 8,804 View
- 163 Download
- 18 Web of Science
- 17 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Intratumoral fibrosis (ITF) is a frequent histologic finding in solid organ tumors. Renal cell carcinoma (RCC) is a highly vascularized tumor with different shapes and degrees of ITF and inflammation. ITF is a poor prognostic factor, especially in breast cancer, and is related to intratumoral necrosis (ITN) and intratumoral inflammation (ITI). However, the significance of ITF in RCC has not been fully studied. In this study, we evaluate the relationships between ITF and other clinicopathologic parameters associated with RCC prognosis.
Methods
ITF was evaluated in 204 clear cell renal cell carcinoma (CCRCC) specimens according to presence and grade of fibrosis, degree of ITI, and presence of ITN. Lysyl oxidase (LOX) expression in tumor cells was also evaluated with clinicopathologic parameters.
Results
Among 204 CCRCC cases, 167 (81.7%) showed ITF, 71 (34.8%) showed ITI, 35 (17.2%) showed ITN, and 111 (54.4%) showed LOX expression. ITF correlated with Fuhrman nuclear grade (p = .046), lymphovascular invasion (LVI) (p = .027), and ITN (p = .036). Patients with ITF had a poor five-year overall survival rate (p = .104).
Conclusions
ITF is related to other poor prognostic factors in CCRCC, such as Fuhrman nuclear grade, ITN, and LVI, but ITF itself had no significant correlation with prognosis of CCRCC. -
Citations
Citations to this article as recorded by- Postoperative prognostic assessment using ECV fraction derived from equilibrium contrast-enhanced CT in thymomas
Koji Takumi, Hiroto Hakamada, Hiroaki Nagano, Ryota Nakanosono, Fumiko Kanzaki, Masanori Nakajo, Kiyohisa Kamimura, Masatoyo Nakajo, Daigo Nagano, Kazuhiro Ueda, Takashi Yoshiura
European Journal of Radiology.2025; 184: 111978. CrossRef - FAP+ fibroblasts orchestrate tumor microenvironment remodeling in renal cell carcinoma with tumor thrombus
Jiacheng Ma, Yan Huang, Jie Chen, Yang Li, Rongyan Yao, Xiubin Li, Qiyang Liang, Xinran Chen, Cheng Peng, Kan Liu, Yuanjun Zhai, Xu Zhang, Xin Ma, Xiaowen Wang, Qingbo Huang, Fuchu He
Nature Communications.2025;[Epub] CrossRef - New insights into fibrotic signaling in renal cell carcinoma
Jiao-Yi Chen, Wai-Han Yiu, Patrick Ming-Kuen Tang, Sydney Chi-Wai Tang
Frontiers in Cell and Developmental Biology.2023;[Epub] CrossRef - Tumor-associated fibrosis impairs the response to immunotherapy
Angha Naik, Andrew Leask
Matrix Biology.2023; 119: 125. CrossRef - Decreased renal expression of PAQR5 is associated with the absence of a nephroprotective effect of progesterone in a rat UUO model
P. A. Abramicheva, D. S. Semenovich, L. D. Zorova, I. B. Pevzner, I. A. Sokolov, V. A. Popkov, E. P. Kazakov, D. B. Zorov, E. Y. Plotnikov
Scientific Reports.2023;[Epub] CrossRef - Intratumoral fibrosis and patterns of immune infiltration in clear cell renal cell carcinoma
Songchen Han, Wenbo Yang, Caipeng Qin, Yiqing Du, Mengting Ding, Huaqi Yin, Tao Xu
BMC Cancer.2022;[Epub] CrossRef - Siglec-F–expressing neutrophils are essential for creating a profibrotic microenvironment in renal fibrosis
Seungwon Ryu, Jae Woo Shin, Soie Kwon, Jiwon Lee, Yong Chul Kim, Yoe-Sik Bae, Yong-Soo Bae, Dong Ki Kim, Yon Su Kim, Seung Hee Yang, Hye Young Kim
Journal of Clinical Investigation.2022;[Epub] CrossRef - The atypical sphingosine 1‐phosphate variant, d16:1 S1P, mediates CTGF induction via S1P2 activation in renal cell carcinoma
Melanie Glueck, Alexander Koch, Robert Brunkhorst, Nerea Ferreiros Bouzas, Sandra Trautmann, Liliana Schaefer, Waltraud Pfeilschifter, Josef Pfeilschifter, Rajkumar Vutukuri
The FEBS Journal.2022; 289(18): 5670. CrossRef - The Synergistic Cooperation between TGF-β and Hypoxia in Cancer and Fibrosis
Pramod Mallikarjuna, Yang Zhou, Maréne Landström
Biomolecules.2022; 12(5): 635. CrossRef - In Vitro Characterization of Renal Drug Transporter Activity in Kidney Cancer
Pedro Caetano-Pinto, Nathanil Justian, Maria Dib, Jana Fischer, Maryna Somova, Martin Burchardt, Ingmar Wolff
International Journal of Molecular Sciences.2022; 23(17): 10177. CrossRef - PD-1 immunobiology in glomerulonephritis and renal cell carcinoma
Colleen S. Curran, Jeffrey B. Kopp
BMC Nephrology.2021;[Epub] CrossRef - Intratumoral Fibrosis in Facilitating Renal Cancer Aggressiveness: Underlying Mechanisms and Promising Targets
Chao Hu, Yufeng Zhao, Xuanchuan Wang, Tongyu Zhu
Frontiers in Cell and Developmental Biology.2021;[Epub] CrossRef - What Mediates Fibrosis in the Tumor Microenvironment of Clear Renal Cell Carcinoma
Wenbo Yang, Caipeng Qin, Jingli Han, Songchen Han, Wenjun Bai, Yiqing Du, Tao Xu
Frontiers in Genetics.2021;[Epub] CrossRef - Kidney Cancer and Chronic Kidney Disease: Too Close for Comfort
Pedro Caetano Pinto, Cindy Rönnau, Martin Burchardt, Ingmar Wolff
Biomedicines.2021; 9(12): 1761. CrossRef - The Significance of Fibrosis Quantification as a Marker in Assessing Pseudo-Capsule Status and Clear Cell Renal Cell Carcinoma Prognosis
Caipeng Qin, Huaqi Yin, Huixin Liu, Feng Liu, Yiqing Du, Tao Xu
Diagnostics.2020; 10(11): 895. CrossRef - The challenges of adoptive cell transfer in the treatment of human renal cell carcinoma
Zuzana Strizova, Jirina Bartunkova, Daniel Smrz
Cancer Immunology, Immunotherapy.2019; 68(11): 1831. CrossRef - Procollagen-lysine, 2-oxoglutarate 5-dioxygenases 1, 2, and 3 are potential prognostic indicators in patients with clear cell renal cell carcinoma
Wen-Hao Xu, Yue Xu, Jun Wang, Xi Tian, Junlong Wu, Fang-Ning Wan, Hong-Kai Wang, Yuan-Yuan Qu, Hai-Liang Zhang, Ding-Wei Ye
Aging.2019; 11(16): 6503. CrossRef
- Postoperative prognostic assessment using ECV fraction derived from equilibrium contrast-enhanced CT in thymomas
- Implication of PHF2 Expression in Clear Cell Renal Cell Carcinoma
- Cheol Lee, Bohyun Kim, Boram Song, Kyung Chul Moon
- J Pathol Transl Med. 2017;51(4):359-364. Published online June 13, 2017
- DOI: https://doi.org/10.4132/jptm.2017.03.16
- 9,090 View
- 168 Download
- 10 Web of Science
- 12 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Clear cell renal cell carcinoma (CCRCC) is presumed to be associated with adipogenic differentiation. Histone modification is known to be important for adipogenesis, and the function of histone demethylase plant homeodomain finger 2 (PHF2) has been noted. In addition, PHF2 may act as a tumor suppressor via epigenetic regulation of p53 and is reported to be reduced in colon cancer and stomach cancer tissues. In this study, we examined PHF2 expression in CCRCC specimens by immunohistochemistry.
Methods
We studied 254 CCRCCs and 56 non-neoplastic renal tissues from patients who underwent radical or partial nephrectomy between 2000 and 2003 at the Seoul National University Hospital. Tissue microarray blocks were prepared, and immunohistochemical staining for PHF2 was performed.
Results
Among 254 CCRCC cases, 150 cases (59.1%) showed high expression and 104 cases (40.1%) showed low expression. High expression of PHF2 was significantly correlated with a low Fuhrman nuclear grade (p < .001), smaller tumor size (p < .001), low overall stage (p = .003), longer cancer-specific survival (p = .002), and progression-free survival (p < .001) of the patients. However, it was not an independent prognostic factor in multivariate analysis adjusted for Fuhrman nuclear grade and overall stage.
Conclusions
Our study showed that low expression of PHF2 is associated with aggressiveness and poor prognosis of CCRCC. -
Citations
Citations to this article as recorded by- The role of histone demethylase PHF2 as a tumour suppressor in hepatocellular carcinoma by regulating SRXN1
Dexter Kai Hao Thng, Lissa Hooi, Wai Khang Yong, Dennis Kappei, Tan Boon Toh, Edward Kai-Hua Chow
Oncogenesis.2026;[Epub] CrossRef - Phosphoproteomics identifies determinants of PAK inhibitor sensitivity in leukaemia cells
Pedro Casado, Santiago Marfa, Marym M. Hadi, Henry Gerdes, Sandra M. Martin-Guerrero, Farideh Miraki-Moud, Vinothini Rajeeve, Pedro R. Cutillas
Cell Communication and Signaling.2025;[Epub] CrossRef - The role of histone methylation in renal cell cancer: an update
Yanguang Hou, Yan Yuan, Yanze Li, Lei Wang, Juncheng Hu, Xiuheng Liu
Molecular Biology Reports.2023; 50(3): 2735. CrossRef - Phosphorylation of PHF2 by AMPK releases the repressive H3K9me2 and inhibits cancer metastasis
Ying Dong, Hao Hu, Xuan Zhang, Yunkai Zhang, Xin Sun, Hanlin Wang, Weijuan Kan, Min-jia Tan, Hong Shi, Yi Zang, Jia Li
Signal Transduction and Targeted Therapy.2023;[Epub] CrossRef - HIF-1α-mediated augmentation of miRNA-18b-5p facilitates proliferation and metastasis in osteosarcoma through attenuation PHF2
Peng Luo, Yan-dong Zhang, Feng He, Chang-jun Tong, Kai Liu, He Liu, Shi-zhuang Zhu, Jian-zhou Luo, Bing Yuan
Scientific Reports.2022;[Epub] CrossRef - Integration of meta-analysis and supervised machine learning for pattern recognition in breast cancer using epigenetic data
Reza Panahi, Esmaeil Ebrahimie, Ali Niazi, Alireza Afsharifar
Informatics in Medicine Unlocked.2021; 24: 100629. CrossRef - PHF2 regulates homology-directed DNA repair by controlling the resection of DNA double strand breaks
Ignacio Alonso-de Vega, Maria Cristina Paz-Cabrera, Magdalena B Rother, Wouter W Wiegant, Cintia Checa-Rodríguez, Juan Ramón Hernández-Fernaud, Pablo Huertas, Raimundo Freire, Haico van Attikum, Veronique A J Smits
Nucleic Acids Research.2020; 48(9): 4915. CrossRef - Emerging of lysine demethylases (KDMs): From pathophysiological insights to novel therapeutic opportunities
Sarder Arifuzzaman, Mst Reshma Khatun, Rabeya Khatun
Biomedicine & Pharmacotherapy.2020; 129: 110392. CrossRef - Biology and targeting of the Jumonji-domain histone demethylase family in childhood neoplasia: a preclinical overview
Tyler S. McCann, Lays M. Sobral, Chelsea Self, Joseph Hsieh, Marybeth Sechler, Paul Jedlicka
Expert Opinion on Therapeutic Targets.2019; 23(4): 267. CrossRef - MiR-221 Promotes Hepatocellular Carcinoma Cells Migration via Targeting PHF2
Yi Fu, Mingyan Liu, Fengxia Li, Li Qian, Ping Zhang, Fengwei Lv, Wenting Cheng, Ruixing Hou
BioMed Research International.2019; 2019: 1. CrossRef - PHF2 histone demethylase prevents DNA damage and genome instability by controlling cell cycle progression of neural progenitors
Stella Pappa, Natalia Padilla, Simona Iacobucci, Marta Vicioso, Elena Álvarez de la Campa, Claudia Navarro, Elia Marcos, Xavier de la Cruz, Marian A. Martínez-Balbás
Proceedings of the National Academy of Sciences.2019; 116(39): 19464. CrossRef - Plant homeodomain finger protein 2 as a novel IKAROS target in acute lymphoblastic leukemia
Zheng Ge, Yan Gu, Qi Han, Justin Sloane, Qinyu Ge, Goufeng Gao, Jinlong Ma, Huihui Song, Jiaojiao Hu, Baoan Chen, Sinisa Dovat, Chunhua Song
Epigenomics.2018; 10(1): 59. CrossRef
- The role of histone demethylase PHF2 as a tumour suppressor in hepatocellular carcinoma by regulating SRXN1
- Clear Cell Renal Cell Carcinoma with Intratumoral Granulomatous Reaction: A Case Report and Review of the Literature
- Hayeon Kim, Jong Wook Kim, Aeree Kim, Hyeyoon Chang
- J Pathol Transl Med. 2017;51(3):325-328. Published online March 14, 2017
- DOI: https://doi.org/10.4132/jptm.2016.09.08
- 9,734 View
- 122 Download
-
 Abstract
Abstract
 PDF
PDF - Granulomatous reaction associated with clear cell renal cell carcinoma (CCRCC) is a rare finding, and only a few cases have been described in the literature. It is postulated to occur due to cancer- related antigenic factors such as cancer cells themselves or soluble tumor antigens shed into the blood. Herein, we describe a case of a 56-year-old male patient diagnosed with CCRCC with intratumoral granulomatous inflammation.
- Oncocytic Renal Cell Carcinoma with Tubulopapillary Growth Having a Fat Component
- Na Rae Kim, Hyun Yee Cho
- J Pathol Transl Med. 2015;49(5):413-417. Published online July 30, 2015
- DOI: https://doi.org/10.4132/jptm.2015.07.01
- 11,442 View
- 82 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF - We report a rare case of oncocytic renal cell carcinoma (RCC) with tubulopapillary growth in the background of tuberculous end-stage kidney disease. Histology of the renal mass consisted of oncocytic cells forming solid, thin tubules and rare papillae. The tumor had abundant eosinophilic oncocytic cells containing occasional cytoplasmic Mallory body–like hyaline globules and a tiny focus of clear cells with intervening mature fat. Both the oncocytic cells and clear cells were immunoreactive for a-methylacyl-CoA racemase, vimentin, pancytokeratin, and CD10, and negative for transcription factor E3, CD15, human melanoma black 45, and c-kit. Mallory body–like hyaline globules were positive for CAM 5.2 and periodic acid–Schiff with or without diastase. Ultrastructurally, the tumor cells had abundant cytoplasmic mitochondria. The present case is a rare case of oncocytic RCC with tubulopapillary growth pattern. The case is unique in that the tumor was mixed with fat component, which is not common in RCC and thus can lead to misdiagnosis.
-
Citations
Citations to this article as recorded by- Oncocytic papillary renal cell carcinoma (OPRCC): 2 case report and literature review
Yanchen Wang, Lihui Guan, Yaming Liu, Yuxuan Liu, Xiaoyan Guo, Yaofei Sun
Frontiers in Oncology.2025;[Epub] CrossRef
- Oncocytic papillary renal cell carcinoma (OPRCC): 2 case report and literature review
- Transglutaminase 2 Expression and Its Prognostic Significance in Clear Cell Renal Cell Carcinoma
- Min Jee Park, Hae Woon Baek, Ye-Young Rhee, Cheol Lee, Jeong Whan Park, Hwal Woong Kim, Kyung Chul Moon
- J Pathol Transl Med. 2015;49(1):37-43. Published online January 15, 2015
- DOI: https://doi.org/10.4132/jptm.2014.10.25
- 11,201 View
- 81 Download
- 16 Web of Science
- 18 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
A few recent studies have demonstrated a possible role of transglutaminase 2 (TG2) in tumorigenesis or progression of renal cell carcinoma (RCC). The aim of this study was to examine TG2 expression and its clinicopathologic significance in a large number of human clear cell RCCs (CCRCCs). Methods: We analyzed 638 CCRCC patients who underwent partial or radical nephrectomy between 1995 and 2005. The expression of TG2 was determined by immunohistochemistry and categorized into four groups, according to staining intensity: negative (0), mild (1+), moderate (2+), and strong (3+). Results: TG2 staining intensity was negative in 8.5% of CCRCC (n=54), 1+ in 32.6% (n=208), 2+ in 50.5% (n=322), and 3+ in 8.5% (n=54). Strong TG2 expression was correlated with high Fuhrman nuclear grade (p=.011), high T category (p=.049), metastasis (p=.043) and male sex (p<.001) but not with N category.The survival analysis showed a significant association between strong TG2 expression and worse overall and cancer-specific survival (p=.027 and p=.010, respectively). On multivariate analysis, strong TG2 expression was a marginally significant prognostic indicator for Fuhrman nuclear grade and TNM staging (p=.054). Conclusions: Our study is the first to demonstrate the clinicopathologic significance of TG2 expression in a large number of human CCRCC samples. Strong TG2 expression was associated with high nuclear grade and poor prognosis. -
Citations
Citations to this article as recorded by- Sex-mediated effects of transglutaminase 2 inhibition on endothelial function in human resistance arteries from diabetic and non-diabetic patients
Khatera Saii, Judit Prat-Duran, Ulf Simonsen, Anders Riegels Knudsen, Jonas Amstrup Funder, Niels Henrik Buus, Estéfano Pinilla
Clinical Science.2025; 139(1): 1. CrossRef - Discovery of novel 1H-benzo[d]imidazole-4,7-dione based transglutaminase 2 inhibitors as p53 stabilizing anticancer agents in renal cell carcinoma
Ga-Ram Kim, Joon Hee Kang, Hyeon Joo Kim, Eunji Im, Jinsu Bae, Woo Sun Kwon, Sun Young Rha, Hyun Cheol Chung, Eun Yi Cho, Soo-Youl Kim, Yong-Chul Kim
Bioorganic Chemistry.2024; 143: 107061. CrossRef - Transglutaminase 2 is associated with adverse colorectal cancer survival and represents a therapeutic target
Patrizia Malkomes, Ilaria Lunger, Elsie Oppermann, Johannes Lorenz, Sara Fatima Faqar-Uz-Zaman, Jiaoyan Han, Sabrina Bothur, Paul Ziegler, Katrin Bankov, Peter Wild, Wolf Otto Bechstein, Michael A. Rieger
Cancer Gene Therapy.2023; 30(10): 1346. CrossRef - Transglutaminase Type 2-MITF axis regulates phenotype switching in skin cutaneous melanoma
Silvia Muccioli, Valentina Brillo, Tatiana Varanita, Federica Rossin, Elisabetta Zaltron, Angelo Velle, Giorgia Alessio, Beatrice Angi, Filippo Severin, Anna Tosi, Manuela D’Eletto, Luca Occhigrossi, Laura Falasca, Vanessa Checchetto, Roberto Ciaccio, Ame
Cell Death & Disease.2023;[Epub] CrossRef - The role of transglutaminase 2 in regulation of the balance between autophagy and apoptosis in tumor cells
Yu. A. Gnennaya, O. M. Semenov, N. A. Barlev
Advances in Molecular Oncology.2023; 10(4): 31. CrossRef - Application of a Fluorescence Anisotropy-Based Assay to Quantify Transglutaminase 2 Activity in Cell Lysates
Sandra Hauser, Paul Sommerfeld, Johanna Wodtke, Christoph Hauser, Paul Schlitterlau, Jens Pietzsch, Reik Löser, Markus Pietsch, Robert Wodtke
International Journal of Molecular Sciences.2022; 23(9): 4475. CrossRef - The Biological and Biomechanical Role of Transglutaminase-2 in the Tumour Microenvironment
Robert Tempest, Sonia Guarnerio, Rawan Maani, Jamie Cooper, Nicholas Peake
Cancers.2021; 13(11): 2788. CrossRef - A Precision Strategy to Cure Renal Cell Carcinoma by Targeting Transglutaminase 2
Soo-Youl Kim, Jeffrey W. Keillor
International Journal of Molecular Sciences.2020; 21(7): 2493. CrossRef - Evaluation of nuclear NF-κB, transglutaminase2, and ERCC1 as predictors of platinum resistance in testicular tumors
Alan A. Azambuja, Paula Engroff, Bruna T. Silva, Roberta C. S. Zorzetti, Fernanda B. Morrone
International braz j urol.2020; 46(3): 353. CrossRef - Transglutaminase 2-Mediated p53 Depletion Promotes Angiogenesis by Increasing HIF-1α-p300 Binding in Renal Cell Carcinoma
Seon-Hyeong Lee, Joon Hee Kang, Ji Sun Ha, Jae-Seon Lee, Su-Jin Oh, Hyun-Jung Choi, Jaewhan Song, Soo-Youl Kim
International Journal of Molecular Sciences.2020; 21(14): 5042. CrossRef - Role of Tissue Transglutaminase Catalytic and Guanosine Triphosphate-Binding Domains in Renal Cell Carcinoma Progression
Burge Ulukan, Ajna Bihorac, Tarik Sipahioglu, Robert Kiraly, Laszlo Fesus, Dilek Telci
ACS Omega.2020; 5(43): 28273. CrossRef - Transglutaminase 2: The Maestro of the Oncogenic Mediators in Renal Cell Carcinoma
Ayca Ece Nezir, Burge Ulukan, Dilek Telci
Medical Sciences.2019; 7(2): 24. CrossRef - Transglutaminase 2 takes center stage as a cancer cell survival factor and therapy target
Richard L. Eckert
Molecular Carcinogenesis.2019; 58(6): 837. CrossRef - Allosteric inhibition site of transglutaminase 2 is unveiled in the N terminus
Nayeon Kim, Joon Hee Kang, Won-Kyu Lee, Seul-Gi Kim, Jae-Seon Lee, Seon-Hyeong Lee, Jong Bae Park, Kyung-Hee Kim, Young-Dae Gong, Kwang Yeon Hwang, Soo-Youl Kim
Amino Acids.2018; 50(11): 1583. CrossRef - Renal Cell Carcinoma Is Abrogated by p53 Stabilization through Transglutaminase 2 Inhibition
Seon-Hyeong Lee, Won-Kyu Lee, Nayeon Kim, Joon Hee Kang, Kyung-Hee Kim, Seul-Gi Kim, Jae-Seon Lee, Soohyun Lee, Jongkook Lee, Jungnam Joo, Woo Sun Kwon, Sun Young Rha, Soo-Youl Kim
Cancers.2018; 10(11): 455. CrossRef - Tissue transglutaminase expression is necessary for adhesion, metastatic potential and cancer stemness of renal cell carcinoma
Yesim Bagatur, Ayca Zeynep Ilter Akulke, Ajna Bihorac, Merve Erdem, Dilek Telci
Cell Adhesion & Migration.2017; : 1. CrossRef - Characterization of clear cell renal cell carcinoma by gene expression profiling
Bryan J. Thibodeau, Matthew Fulton, Laura E. Fortier, Timothy J. Geddes, Barbara L. Pruetz, Samreen Ahmed, Amy Banes-Berceli, Ping L. Zhang, George D. Wilson, Jason Hafron
Urologic Oncology: Seminars and Original Investigations.2016; 34(4): 168.e1. CrossRef - Prognostic role of tissue transglutaminase 2 in colon carcinoma
María Jesús Fernández-Aceñero, Sofía Torres, Irene Garcia-Palmero, Cristina Díaz del Arco, J. Ignacio Casal
Virchows Archiv.2016; 469(6): 611. CrossRef
- Sex-mediated effects of transglutaminase 2 inhibition on endothelial function in human resistance arteries from diabetic and non-diabetic patients
- IMP3, a Promising Prognostic Marker in Clear Cell Renal Cell Carcinoma
- Ji Young Park, Misun Choe, Yuna Kang, Sang Sook Lee
- Korean J Pathol. 2014;48(2):108-116. Published online April 28, 2014
- DOI: https://doi.org/10.4132/KoreanJPathol.2014.48.2.108
- 9,138 View
- 45 Download
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF Background Insulin-like growth factor II mRNA-binding protein 3 (IMP3) has been reported as a prognostic biomarker in various cancers. To validate IMP3 as a prognostic biomarker in renal cell carcinoma (RCC), we investigated the expression of IMP3, p53, and Ki-67, and their associations with clinicopathologic outcomes.
Methods We studied 148 clear cell RCCs (CCRCCs) from patients who underwent radical nephrectomy. The expression levels of IMP3, p53, and Ki-67 were assessed by immunohistochemical staining and the clinical and pathologic parameters were retrospectively reviewed.
Results Twenty-nine percent of CCRCCs expressed IMP3. Forty-one percent of IMP3-immunopositive tumors developed metastases, while only 11.4% of IMP3-negative tumors developed metastases (p<.001). A Kaplan-Meier curve showed that patients with IMP3-immunopositive tumors had lower metastasis-free survival and cancer-specific survival than did those with IMP3-immunonegative tumors (p<.001 and p<.001, respectively). Expression of high Ki-67 proliferation index was also associated with a higher metastatic rate. In the multivariate Cox regression analysis, pT stage and IMP3-positivity were independently associated with disease-specific survival.
Conclusions IMP3 is an independent prognostic biomarker for patients with CCRCC to predict metastasis and poor outcome.
-
Citations
Citations to this article as recorded by- IMP3 Immunohistochemical Expression Is Related with Progression and Metastases in Xenografted and Cutaneous Melanomas
Natividad Martin-Morales, Miguel Padial-Molina, Isabel Tovar, Virginea De Araujo Farias, Pedro Hernández-Cortés, Esperanza Ramirez-Moreno, Mercedes Caba-Molina, Justin Davis, Alejandro Carrero Castaño, Jose Mariano Ruiz de Almodovar, Pablo Galindo-Moreno,
Pathobiology.2024; 91(2): 132. CrossRef - circRARS synergises with IGF2BP3 to regulate RNA methylation recognition to promote tumour progression in renal cell carcinoma
Yuenan Liu, Kailei Chen, Yi Shou, Sen Li, Jun Wang, Qingyang Zhang, Ziwei Huang, Jiaju Xu, Mingfeng Li, Di Liu, Huageng Liang, Hongmei Yang, Xiaoping Zhang
Clinical and Translational Medicine.2023;[Epub] CrossRef - Prognostic value of insulin‑like growth factor 2 mRNA‑binding protein 3 and vascular endothelial growth factor‑A in patients with primary non‑small‑cell lung cancer
Jiannan Liu, Ying Liu, Wenjing Gong, Xiangshuo Kong, Congcong Wang, Shuhua Wang, Aina Liu
Oncology Letters.2019;[Epub] CrossRef - Epithelial‑mesenchymal transition in colorectal carcinoma cells is mediated by DEK/IMP3
Shuping You, Yun Guan, Weihong Li
Molecular Medicine Reports.2017;[Epub] CrossRef
- IMP3 Immunohistochemical Expression Is Related with Progression and Metastases in Xenografted and Cutaneous Melanomas
- Renal Histologic Parameters Influencing Postoperative Renal Function in Renal Cell Carcinoma Patients
- Myoung Ju Koh, Beom Jin Lim, Kyu Hun Choi, Yon Hee Kim, Hyeon Joo Jeong
- Korean J Pathol. 2013;47(6):557-562. Published online December 24, 2013
- DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.6.557
- 7,649 View
- 45 Download
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF Background Pre-existing non-neoplastic renal diseases or lesions may influence patient renal function after tumor removal. However, its description is often neglected or omitted in pathologic reports. To determine the incidence and clinical significance of non-neoplastic lesions, we retrospectively examined renal tissues obtained during 85 radical nephrectomies for renal cell carcinoma.
Methods One paraffin-embedded tissue block from each case containing a sufficient amount of non-tumorous renal parenchyma was cut and processed with hematoxylin and eosin and periodic acid-Schiff methods. Non-neoplastic lesions of each histological compartment were semi-quantitatively and quantitatively evaluated.
Results Among the various histologic lesions found, tubular atrophy, arterial intimal thickening, and glomerulosclerosis were the most common (94.1%, 91.8%, and 88.2%, respectively). Glomerulosclerosis correlated with estimated glomerular filtration rate at the time of surgery, as well as at 1- and 5-years post-surgery (p=.0071), but tubulointerstitial fibrosis or arterial fibrous intimal thickening did not.
Post-hoc analysis revealed that glomerulosclerosis of more than 20% predicted post-operative renal function. However, its significance disappeared when gender and age were considered.Conclusions In conclusion, non-neoplastic lesions, especially with regard to glomerulosclerosis percentage, should be described in pathology reports to provide additional information on renal function decline.
-
Citations
Citations to this article as recorded by- Diffusion kurtosis versus diffusion-weighted magnetic resonance imaging in differentiating clear cell renal cell carcinoma and renal angiomyolipoma with minimal fat: a comparative study
Yarong Lin, Wenrong Zhu, Qingqiang Zhu
Diagnostic and Interventional Radiology.2025;[Epub] CrossRef - Role of intravoxel incoherent motion diffusion-weighted MRI in differentiation of renal cell carcinoma subtypes
Amira R. Mahmoud, Nehad Fouda, Eman Mohamed Helmy, Ali Elsorougy
Egyptian Journal of Radiology and Nuclear Medicine.2024;[Epub] CrossRef - Chronic kidney damage pathology score for systematic assessment of the non-neoplastic kidney tissue and prediction of post-operative renal function outcomes
Yong Jia, Seyed M.M. Poor, Brenden Dufault, Vivian Lu, Jasmir G. Nayak, Deepak K. Pruthi, Ian W. Gibson
Human Pathology.2022; 124: 76. CrossRef - Value of intravoxel incoherent motion for differential diagnosis of renal tumors
Qingqiang Zhu, Wenrong Zhu, Jing Ye, Jingtao Wu, Wenxin Chen, Zhihua Hao
Acta Radiologica.2019; 60(3): 382. CrossRef - Conventional and Papillary Renal Cell Carcinomas and Focal Segmental Glomerulosclerosis in a Nephrectomy
Firas Al-Delfi, Guillermo A. Herrera
Pathology Case Reviews.2015; 20(6): 263. CrossRef
- Diffusion kurtosis versus diffusion-weighted magnetic resonance imaging in differentiating clear cell renal cell carcinoma and renal angiomyolipoma with minimal fat: a comparative study
- ALK-Positive Renal Cell Carcinoma in a Large Series of Consecutively Resected Korean Renal Cell Carcinoma Patients
- Cheol Lee, Jeong Whan Park, Ja Hee Suh, Kyung Han Nam, Kyung Chul Moon
- Korean J Pathol. 2013;47(5):452-457. Published online October 25, 2013
- DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.5.452
- 9,659 View
- 67 Download
- 31 Crossref
-
 Abstract
Abstract
 PDF
PDF Background Recently, there have been a few reports of renal cell carcinoma (RCC) cases with anaplastic lymphoma kinase (
ALK ) gene fusion. In this study, we screened consecutively resected RCCs from a single institution for ALK protein expression by immunohistochemistry, and then we performed fluorescencein situ hybridization to confirm theALK gene alteration in ALK immunohistochemistry-positive cases.Methods We screened 829 RCCs by ALK immunohistochemistry, and performed fluorescence
in situ hybridization analysis usingALK dual-color break-apart rearrangement probe. Histological review and additional immunohistochemistry analyses were done in positive cases.Results One ALK-positive case was found. Initial diagnosis of this case was papillary RCC type 2. This comprises 0.12% of all RCCs (1/829) and 1.9% of papillary RCCs (1/53). This patient was a 44-year-old male with RCC found during routine health check-up. He was alive without evidence of disease 12 years after surgery. The tumor showed a papillary and tubular pattern, and showed positivity for CD10 (focal), epithelial membrane antigen, cytokeratin 7, pan-cytokeratin, PAX-2, and vimentin.
Conclusions We found the first RCC case with
ALK gene rearrangement in Korean patients by ALK immunohistochemistry among 829 RCCs. This case showed similar histological and immunohistochemical features to those of previous adult cases withALK rearrangement, and showed relatively good prognosis.-
Citations
Citations to this article as recorded by- Renal cell carcinoma with ALK-TPM3 gene fusion and ALK amplification: A case report and literature review
Xinzhuo Tu, Min Zhu, Qingyue Liu, Xu Liu, Yayun Qi, Yuanlin Zhang, Haili Li, Tianzhu Tao, Jinjin Chang, Jianping Zhu, Dawei Mu, Li Ren, Dengfeng Cao, Teng Li
Pathology - Research and Practice.2025; 266: 155814. CrossRef -
ALK-Rearranged Renal Cell Carcinoma: A Case Report with Review of Literature
Gauri Deshpande, Amandeep Arora, Aparna Katdare, Gagan Prakash, Amit Joshi, Vedang Murthy, Sangeeta Desai, Santosh Menon
Indian Journal of Medical and Paediatric Oncology.2025;[Epub] CrossRef - Research Progress of ALK-Rearranged Renal Cell Carcinoma
瑞珂 王
Advances in Clinical Medicine.2025; 15(12): 746. CrossRef - ALK-Rearranged Renal Cell Carcinoma: A Multi-Institutional Study of 9 Cases With Expanding the Morphologic and Molecular Genetic Spectrum
Ming Zhao, Xiaona Yin, Xiaoqun Yang, Hualei Gan, Ni Chen, Guangjie Duan, Yanfeng Bai, Xiaodong Teng, Jiayun Xu, Rong Fang, Suying Wang, Shan Zhong, Xiaotong Wang, Lisong Teng
Modern Pathology.2024; 37(8): 100536. CrossRef - Activity of ALK Inhibitors in Renal Cancer with ALK Alterations: A Systematic Review
Giovanni Maria Iannantuono, Silvia Riondino, Stefano Sganga, Mario Roselli, Francesco Torino
International Journal of Molecular Sciences.2022; 23(7): 3995. CrossRef - Novel, emerging and provisional renal entities: The Genitourinary Pathology Society (GUPS) update on renal neoplasia
Kiril Trpkov, Sean R. Williamson, Anthony J. Gill, Adebowale J. Adeniran, Abbas Agaimy, Reza Alaghehbandan, Mahul B. Amin, Pedram Argani, Ying-Bei Chen, Liang Cheng, Jonathan I. Epstein, John C. Cheville, Eva Comperat, Isabela Werneck da Cunha, Jennifer B
Modern Pathology.2021; 34(6): 1167. CrossRef - ESC, ALK, HOT and LOT: Three Letter Acronyms of Emerging Renal Entities Knocking on the Door of the WHO Classification
Farshid Siadat, Kiril Trpkov
Cancers.2020; 12(1): 168. CrossRef - ALK-rearranged renal cell carcinoma with a novel PLEKHA7-ALK translocation and metanephric adenoma-like morphology
Jen-Fan Hang, Hsiao-Jen Chung, Chin-Chen Pan
Virchows Archiv.2020; 476(6): 921. CrossRef - Characteristics of Renal Cell Carcinoma Harboring TPM3-ALK Fusion
Chang Gok Woo, Seok Jung Yun, Seung-Myoung Son, Young Hyun Lim, Ok-Jun Lee
Yonsei Medical Journal.2020; 61(3): 262. CrossRef - Report From the International Society of Urological Pathology (ISUP) Consultation Conference on Molecular Pathology of Urogenital Cancers
Sean R. Williamson, Anthony J. Gill, Pedram Argani, Ying-Bei Chen, Lars Egevad, Glen Kristiansen, David J. Grignon, Ondrej Hes
American Journal of Surgical Pathology.2020; 44(7): e47. CrossRef - ALK rearranged renal cell carcinoma (ALK-RCC): a multi-institutional study of twelve cases with identification of novel partner genes CLIP1, KIF5B and KIAA1217
Naoto Kuroda, Kiril Trpkov, Yuan Gao, Maria Tretiakova, Yajuan J. Liu, Monika Ulamec, Kengo Takeuchi, Abbas Agaimy, Christopher Przybycin, Cristina Magi-Galluzzi, Soichiro Fushimi, Fumiyoshi Kojima, Malthide Sibony, Jen-Fan Hang, Chin-Chen Pan, Asli Yilma
Modern Pathology.2020; 33(12): 2564. CrossRef - ALK rearrangement in TFE3-positive renal cell carcinoma: Alternative diagnostic option to exclude Xp11.2 translocation carcinoma
Yiqi Zhu, Ning Liu, Wei Guo, Xiaohong Pu, Hongqian Guo, Weidong Gan, Dongmei Li
Pathology - Research and Practice.2020; 216(12): 153286. CrossRef - New and emerging renal entities: a perspective post‐WHO 2016 classification
Kiril Trpkov, Ondřej Hes
Histopathology.2019; 74(1): 31. CrossRef - Lack of expression of ALK and CD30 in breast carcinoma by immunohistochemistry irrespective of tumor characteristics
Samer Nassif, Ziad M. El-Zaatari, Michel Attieh, Maya Hijazi, Najla Fakhreddin, Tarek Aridi, Fouad Boulos
Medicine.2019; 98(32): e16702. CrossRef - Targeted next-generation sequencing revealed distinct clinicopathologic and molecular features of VCL-ALK RCC: A unique case from an older patient without clinical evidence of sickle cell trait
Xiao-tong Wang, Ru Fang, Sheng-bing Ye, Ru-song Zhang, Rui Li, Xuan Wang, Rong-hao Ji, Zhen-feng Lu, Heng-hui Ma, Xiao-jun Zhou, Qiu-yuan Xia, Qiu Rao
Pathology - Research and Practice.2019; 215(11): 152651. CrossRef - ALK-rearranged renal cell carcinomas in Polish population
Adam Gorczynski, Piotr Czapiewski, Aleksandra Korwat, Lukasz Budynko, Monika Prelowska, Krzysztof Okon, Wojciech Biernat
Pathology - Research and Practice.2019; 215(12): 152669. CrossRef - ALK-TPM3 rearrangement in adult renal cell carcinoma: a case report and literature review
Jing Yang, Lei Dong, Hong Du, Xiu-bo Li, Yan-xiao Liang, Guo-rong Liu
Diagnostic Pathology.2019;[Epub] CrossRef - Molecular Genetics of Renal Cell Tumors: A Practical Diagnostic Approach
Reza Alaghehbandan, Delia Perez Montiel, Ana Silvia Luis, Ondrej Hes
Cancers.2019; 12(1): 85. CrossRef - ALK-TPM3 rearrangement in adult renal cell carcinoma: Report of a new case showing loss of chromosome 3 and literature review
Yohan Bodokh, Damien Ambrosetti, Valérie Kubiniek, Branwel Tibi, Matthieu Durand, Jean Amiel, Morgane Pertuit, Anne Barlier, Florence Pedeutour
Cancer Genetics.2018; 221: 31. CrossRef - Prognostic implications of polycomb proteins ezh2, suz12, and eed1 and histone modification by H3K27me3 in sarcoma
Yong Jin Cho, Soo Hee Kim, Eun Kyung Kim, Jung Woo Han, Kyoo-Ho Shin, Hyuk Hu, Kyung Sik Kim, Young Deuk Choi, Sunghoon Kim, Young Han Lee, Jin-Suck Suh, Joong Bae Ahn, Hyun Cheol Chung, Sung Hoon Noh, Sun Young Rha, Sung-Taek Jung, Hyo Song Kim
BMC Cancer.2018;[Epub] CrossRef - Responses to Alectinib in ALK-rearranged Papillary Renal Cell Carcinoma
Sumanta K. Pal, Paulo Bergerot, Nazli Dizman, Cristiane Bergerot, Jacob Adashek, Russell Madison, Jon H. Chung, Siraj M. Ali, Jeremy O. Jones, Ravi Salgia
European Urology.2018; 74(1): 124. CrossRef - Genetic analysis and clinicopathological features of ALK‐rearranged renal cell carcinoma in a large series of resected Chinese renal cell carcinoma patients and literature review
Wenjuan Yu, Yuewei Wang, Yanxia Jiang, Wei Zhang, Yujun Li
Histopathology.2017; 71(1): 53. CrossRef - A case of anaplastic lymphoma kinase‐positive renal cell carcinoma coincident with Hodgkin lymphoma
Yuzo Oyama, Haruto Nishida, Takahiro Kusaba, Hiroko Kadowaki, Motoki Arakane, Tsutomu Daa, Dai Watanabe, Yasuyuki Akita, Fuminori Sato, Hiromitsu Mimata, Shigeo Yokoyama
Pathology International.2017; 67(12): 626. CrossRef - Clinicopathologic and Molecular Pathology of Collecting Duct Carcinoma and Related Renal Cell Carcinomas
An Na Seo, Ghilsuk Yoon, Jae Y. Ro
Advances in Anatomic Pathology.2017; 24(2): 65. CrossRef - The role of the polycomb repressive complex pathway in T and NK cell lymphoma: biological and prognostic implications
Soo Hee Kim, Woo Ick Yang, Yoo Hong Min, Young Hyeh Ko, Sun Och Yoon
Tumor Biology.2016; 37(2): 2037. CrossRef - New and emerging renal tumour entities
Naoto Kuroda, Ondřej Hess, Ming Zhou
Diagnostic Histopathology.2016; 22(2): 47. CrossRef - ALK‐rearranged renal cell carcinomas in children
Mariana M. Cajaiba, Lawrence J. Jennings, Stephen M. Rohan, Antonio R. Perez‐Atayde, Adrian Marino‐Enriquez, Jonathan A. Fletcher, James I. Geller, Katrin M. C. Leuer, Julia A. Bridge, Elizabeth J. Perlman
Genes, Chromosomes and Cancer.2016; 55(5): 442. CrossRef - Two Cases of Renal Cell Carcinoma Harboring a Novel STRN-ALK Fusion Gene
Hironori Kusano, Yuki Togashi, Jun Akiba, Fukuko Moriya, Katsuyoshi Baba, Naomi Matsuzaki, Yoshiaki Yuba, Yusuke Shiraishi, Hiroshi Kanamaru, Naoto Kuroda, Seiji Sakata, Kengo Takeuchi, Hirohisa Yano
American Journal of Surgical Pathology.2016; 40(6): 761. CrossRef - Expanding the spectrum of ALK‐rearranged renal cell carcinomas in children: Identification of a novel HOOK1‐ALK fusion transcript
Mariana M. Cajaiba, Lawrence J. Jennings, David George, Elizabeth J. Perlman
Genes, Chromosomes and Cancer.2016; 55(10): 814. CrossRef - TFE3-positive renal cell carcinomas are not always Xp11 translocation carcinomas: Report of a case with a TPM3-ALK translocation
Paul Scott Thorner, Mary Shago, Paula Marrano, Furqan Shaikh, Gino R. Somers
Pathology - Research and Practice.2016; 212(10): 937. CrossRef - ALK rearrangements-associated renal cell carcinoma (RCC) with unique pathological features in an adult
Marie Jeanneau, Valerie Gregoire, Claude Desplechain, Fabienne Escande, Dan Petre Tica, Sebastien Aubert, Xavier Leroy
Pathology - Research and Practice.2016; 212(11): 1064. CrossRef
- Renal cell carcinoma with ALK-TPM3 gene fusion and ALK amplification: A case report and literature review
- Histologic Variations and Immunohistochemical Features of Metastatic Clear Cell Renal Cell Carcinoma
- Cheol Lee, Jeong-Whan Park, Ja Hee Suh, Kyung Han Nam, Kyung Chul Moon
- Korean J Pathol. 2013;47(5):426-432. Published online October 25, 2013
- DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.5.426
- 12,942 View
- 89 Download
- 17 Crossref
-
 Abstract
Abstract
 PDF
PDF Background Due to advancements in treatment of metastatic and advanced renal cell carcinoma (RCC), it has become increasingly important to diagnose metastatic RCC and the specific subtype. In this study, we investigated the diverse histologic features of metastatic clear cell renal cell carcinoma (CCRCC) cases in comparison with corresponding primary lesions.
Methods We identified 119 metastatic CCRCC cases from 81 corresponding primary lesions diagnosed between 1995 and 2010 and evaluated the diverse histologic and immunohistochemical features of these lesions.
Results A total of 44 primary lesions (54.3%) had a non-clear cell component in addition to a typical clear cell component. Of the 119 metastatic lesions, 63 lesions (52.9%) contained a non-clear cell component, and 29 metastatic lesions were composed of a non-clear cell component only. Rhabdoid features were the most frequent non-clear cell histology among the metastatic lesions. Metastatic CCRCCs mainly showed positive CD10 and epithelial membrane antigen staining and negative cytokeratin 7 staining.
Conclusions Metastatic CCRCC commonly showed a variety of histologic features. If there is a difficulty to diagnose metastatic CCRCC due to a variety of histologic features or small biopsy specimen, histologic review of the primary lesion and immunohistochemical analysis can help determine the correct diagnosis.
-
Citations
Citations to this article as recorded by- Sarcomatoid and Rhabdoid Renal Cell Carcinoma
Adebowale J. Adeniran, Brian Shuch, Peter A. Humphrey
American Journal of Surgical Pathology.2024; 48(7): e65. CrossRef - Emerging Antibody-Drug Conjugate Therapies and Targets for Metastatic Renal Cell Carcinoma
Harrison C. Gottlich, Reza Nabavizadeh, Mihai Dumbrava, Rodrigo Rodrigues Pessoa, Ahmed M. Mahmoud, Ishita Garg, Jacob Orme, Brian A. Costello, John Cheville, Fabrice Lucien
Kidney Cancer.2023; 7(1): 161. CrossRef - Painful, bleeding fingertip papule
Jane Gay, Sarah Simpson, Patrick Rush, Alex Holliday
JAAD Case Reports.2022; 21: 130. CrossRef - Development and initial clinical testing of a multiplexed circulating tumor cell assay in patients with clear cell renal cell carcinoma
Rory M. Bade, Jennifer L. Schehr, Hamid Emamekhoo, Benjamin K. Gibbs, Tamara S. Rodems, Matthew C. Mannino, Joshua A. Desotelle, Erika Heninger, Charlotte N. Stahlfeld, Jamie M. Sperger, Anupama Singh, Serena K. Wolfe, David J. Niles, Waddah Arafat, John
Molecular Oncology.2021; 15(9): 2330. CrossRef - Laparoscopic cytoreductive nephrectomy and adrenalectomy for metachronous RCC metastases—Case report
Bogdan Petrut, Cristina Eliza Bujoreanu, Vasile Vlad Hardo, Adrian Barbos, Bogdan Fetica
International Journal of Surgery Case Reports.2020; 74(C): 268. CrossRef - Does CARMENA mark the end of cytoreductive nephrectomy for metastatic renal cell carcinoma?
Steven L. Chang, Toni K. Choueiri, Lauren C. Harshman
Urologic Oncology: Seminars and Original Investigations.2019; 37(8): 525. CrossRef - Metastatic TFE3-overexpressing renal clear cell carcinoma with dense granules: a histological, immunohistochemical, and ultrastructural study
Shoujun Chen, Elba A. Turbat-Herrera, Guillermo A. Herrera, Meghna Chadha, Rodney E. Shackelford, Eric X. Wei
Ultrastructural Pathology.2018; 42(4): 369. CrossRef - The Clinical Activity of PD-1/PD-L1 Inhibitors in Metastatic Non–Clear Cell Renal Cell Carcinoma
Rana R. McKay, Dominick Bossé, Wanling Xie, Stephanie A.M. Wankowicz, Abdallah Flaifel, Raphael Brandao, Aly-Khan A. Lalani, Dylan J. Martini, Xiao X. Wei, David A. Braun, Eliezer Van Allen, Daniel Castellano, Guillermo De Velasco, J. Connor Wells, Daniel
Cancer Immunology Research.2018; 6(7): 758. CrossRef - Implication of PHF2 Expression in Clear Cell Renal Cell Carcinoma
Cheol Lee, Bohyun Kim, Boram Song, Kyung Chul Moon
Journal of Pathology and Translational Medicine.2017; 51(4): 359. CrossRef - Pulmonary metastasectomy from renal cell carcinoma including 3 cases with sarcomatoid component
Tsuyoshi Ueno, Motohiro Yamashita, Shigeki Sawada, Ryujiro Sugimoto, Noriko Nishijima, Yoshifumi Sugawara, Iku Ninomiya
General Thoracic and Cardiovascular Surgery.2016; 64(3): 149. CrossRef - Are primary renal cell carcinoma and metastases of renal cell carcinoma the same cancer?
Aleksandra Semeniuk-Wojtaś, Rafał Stec, Cezary Szczylik
Urologic Oncology: Seminars and Original Investigations.2016; 34(5): 215. CrossRef - Concordance of Pathologic Features Between Metastatic Sites and the Primary Tumor in Surgically Resected Metastatic Renal Cell Carcinoma
Sarah P. Psutka, John C. Cheville, Brian A. Costello, Suzanne B. Stewart-Merrill, Christine M. Lohse, Bradley C. Leibovich, Stephen A. Boorjian, R. Houston Thompson
Urology.2016; 96: 106. CrossRef - The Correlation of Tissue-Based Biomarkers in Primary and Metastatic Renal Cell Carcinoma Lesions: A Tissue Microarray Study
Sung Han Kim, Weon Seo Park, Eun Young Park, Boram Park, Jungnam Joo, Jae Young Joung, Ho Kyung Seo, Kang Hyun Lee, Jinsoo Chung
The Korean Journal of Urological Oncology.2016; 14(3): 152. CrossRef - Long-term follow-up and clinical course of a rare case of von Hippel-Lindau disease: A case report and review of the literature
YU ZOU, JINGJING XU, MINMING ZHANG
Oncology Letters.2016; 11(5): 3273. CrossRef - Genetic alterations in renal cell carcinoma with rhabdoid differentiation
Carmen M. Perrino, Vishwanathan Hucthagowder, Michael Evenson, Shashikant Kulkarni, Peter A. Humphrey
Human Pathology.2015; 46(1): 9. CrossRef - High expression of APRIL correlates with poor prognosis in clear cell renal cell carcinoma
Cheol Lee, Jeong-Whan Park, Ja Hee Suh, Kyung Chul Moon
Pathology - Research and Practice.2015; 211(11): 824. CrossRef - A Case of Cutaneous Metastasis from a Clear Cell Renal Cell Carcinoma with an Eosinophilic Cell Component to the Submandibular Region
Yusuke Amano, Sumie Ohni, Toshiyuki Ishige, Taku Homma, Tsutomu Yamada, Nobuyuki Nishimori, Norimichi Nemoto
Journal of Nihon University Medical Association.2015; 74(2): 73. CrossRef
- Sarcomatoid and Rhabdoid Renal Cell Carcinoma
- Clear Cell Papillary Renal Cell Carcinoma: A Report of 15 Cases Including Three Cases of Concurrent Other-Type Renal Cell Carcinomas
- Jeong Hwan Park, Cheol Lee, Ja Hee Suh, Kyung Chul Moon
- Korean J Pathol. 2012;46(6):541-547. Published online December 26, 2012
- DOI: https://doi.org/10.4132/KoreanJPathol.2012.46.6.541
- 9,856 View
- 65 Download
- 23 Crossref
-
 Abstract
Abstract
 PDF
PDF Background Clear cell papillary renal cell carcinoma (CCPRCC) is a recently established subtype of renal epithelial tumor. The aim of this study was to identify the diagnostic criteria of CCPRCC with an emphasis on immunohistochemical studies, and to report three cases with concurrent other-type renal cell carcinoma (RCC).
Methods A total of 515 RCC patients that consecutively underwent surgical resection at Seoul National University Hospital from 1 January 2010 to 31 December 2011 were screened. Each case was reviewed based on the histologic features and was evaluated immunohistochemically.
Results A total of 15 CCPRCCs were identified, which composed 2.9% of the total RCCs. The mean age was 52 years, and the average tumor size was 1.65 cm. All 15 cases showed low nuclear grade, no lymph node metastasis and no distant metastasis. The CCPRCCs showed variable architectural patterns including cystic, trabecular, papillary, and acinar. All of the cases showed moderate to intense immunoreactivity for cytokeratin 7 (CK7). CD10 was negative or showed focal weak positivity. Three cases had concurrent other-type RCC, including a clear cell RCC and an acquired cystic disease-associated RCC.
Conclusions The strong CK7 and negative or focal weak CD10 expression will be useful for the diagnosis of CCPRCC.
-
Citations
Citations to this article as recorded by- Vascular, adipose tissue, and/or calyceal invasion in clear cell tubulopapillary renal cell tumour: potentially problematic diagnostic scenarios
Ankur R Sangoi, Harrison Tsai, Lara Harik, Jonathan Mahlow, Maria Tretiakova, Sean R Williamson, Michelle S Hirsch
Histopathology.2024; 84(7): 1167. CrossRef - Clinical features and Surgical Outcome of Clear Cell Papillary Renal Cell Tumor: result from a prospective cohort
Si Hyun Kim, Jang Hee Han, Seung-hwan Jeong, Hyeong Dong Yuk, Ja Hyeon Ku, Cheol Kwak, Hyeon Hoe Kim, Kyung Chul Moon, Chang Wook Jeong
BMC Urology.2023;[Epub] CrossRef - Coexistence of multiple clear cell papillary renal cell carcinoma with renal oncocytoma: a case report
Amine Hermi, Ahmed Saadi, Seif Mokadem, Ahlem Blel, Marouene Chakroun, Mohamed Riadh Ben Slama
Annals of Medicine & Surgery.2023; 85(5): 2017. CrossRef - Renal Cell Carcinoma in End-Stage Renal Disease: A Review and Update
Ziad M. El-Zaatari, Luan D. Truong
Biomedicines.2022; 10(3): 657. CrossRef - The Clinicopathologic and Molecular Landscape of Clear Cell Papillary Renal Cell Carcinoma: Implications in Diagnosis and Management
Stanley Weng, Renzo G. DiNatale, Andrew Silagy, Roy Mano, Kyrollis Attalla, Mahyar Kashani, Kate Weiss, Nicole E. Benfante, Andrew G. Winer, Jonathan A. Coleman, Victor E. Reuter, Paul Russo, Ed Reznik, Satish K. Tickoo, A. Ari Hakimi
European Urology.2021; 79(4): 468. CrossRef - Clear cell papillary renal cell carcinoma: Characteristics and survival outcomes from a large single institutional series
James E. Steward, Sean Q. Kern, Liang Cheng, Ronald S. Boris, Yan Tong, Clint D. Bahler, Timothy A. Masterson, K. Clint Cary, Hristos Kaimakliotis, Thomas Gardner, Chandru P. Sundaram
Urologic Oncology: Seminars and Original Investigations.2021; 39(6): 370.e21. CrossRef - Clear cell papillary renal cell carcinoma: an update after 15 years
Sean R. Williamson
Pathology.2021; 53(1): 109. CrossRef - Clear Cell Papillary Renal Cell Carcinoma
Jianping Zhao, Eduardo Eyzaguirre
Archives of Pathology & Laboratory Medicine.2019; 143(9): 1154. CrossRef - Clear cell papillary renal cell carcinoma – An indolent subtype of renal tumor
Wei-Jen Chen, Chin-Chen Pan, Shu-Huei Shen, Hsiao-Jen Chung, Chih-Chieh Lin, Alex T.L. Lin, Yen-Hwa Chang
Journal of the Chinese Medical Association.2018; 81(10): 878. CrossRef - Clear cell papillary renal cell carcinoma: A case report and review of the literature
Sung Han Kim, Whi-An Kwon, Jae Young Joung, Ho Kyung Seo, Kang Hyun Lee, Jinsoo Chung
World Journal of Nephrology.2018; 7(8): 155. CrossRef - Clinical features and survival analysis of clear cell papillary renal cell carcinoma: A 10‑year retrospective study from two institutions
Yiqiu Wang, Ying Ding, Jian Wang, Min Gu, Zengjun Wang, Chao Qin, Conghui Han, Hongxia Li, Xia Liu, Pengfei Wu, Guangchao Li
Oncology Letters.2018;[Epub] CrossRef - A contemporary series of renal masses with emphasis on recently recognized entities and tumors of low malignant potential: A report based on 624 consecutive tumors from a single tertiary center
Maria Rosaria Raspollini, Ilaria Montagnani, Rodolfo Montironi, Liang Cheng, Guido Martignoni, Andrea Minervini, Sergio Serni, Giulio Nicita, Marco Carini, Antonio Lopez-Beltran
Pathology - Research and Practice.2017; 213(7): 804. CrossRef - Renal Neoplasms With Overlapping Features of Clear Cell Renal Cell Carcinoma and Clear Cell Papillary Renal Cell Carcinoma
Hari P. Dhakal, Jesse K. McKenney, Li Yan Khor, Jordan P. Reynolds, Cristina Magi-Galluzzi, Christopher G. Przybycin
American Journal of Surgical Pathology.2016; 40(2): 141. CrossRef - New and emerging renal tumour entities
Naoto Kuroda, Ondřej Hess, Ming Zhou
Diagnostic Histopathology.2016; 22(2): 47. CrossRef - Immunohistochemical Panel for Differentiating Renal Cell Carcinoma with Clear and Papillary Features
Hanan AlSaeid Alshenawy
Pathology & Oncology Research.2015; 21(4): 893. CrossRef - Immunohistochemical panel for differentiating renal cell carcinoma with clear and papillary features
Hanan AlSaeid Alshenawy
Journal of Microscopy and Ultrastructure.2015; 3(2): 68. CrossRef - Clear Cell-Papillary Renal Cell Carcinoma of the Kidney Not Associated With End-stage Renal Disease
Manju Aron, Elena Chang, Loren Herrera, Ondrej Hes, Michelle S. Hirsch, Eva Comperat, Philippe Camparo, Priya Rao, Maria Picken, Michal Michal, Rodolfo Montironi, Pheroze Tamboli, Federico Monzon, Mahul B. Amin
American Journal of Surgical Pathology.2015; 39(7): 873. CrossRef - Papillary or pseudopapillary tumors of the kidney
Fang-Ming Deng, Max X. Kong, Ming Zhou
Seminars in Diagnostic Pathology.2015; 32(2): 124. CrossRef - Do Clear Cell Papillary Renal Cell Carcinomas Have Malignant Potential?
Mairo L. Diolombi, Liang Cheng, Pedram Argani, Jonathan I. Epstein
American Journal of Surgical Pathology.2015; 39(12): 1621. CrossRef - Targeted next‐generation sequencing and non‐coding RNA expression analysis of clear cell papillary renal cell carcinoma suggests distinct pathological mechanisms from other renal tumour subtypes
Charles H Lawrie, Erika Larrea, Gorka Larrinaga, Ibai Goicoechea, María Arestin, Marta Fernandez‐Mercado, Ondrej Hes, Francisco Cáceres, Lorea Manterola, José I López
The Journal of Pathology.2014; 232(1): 32. CrossRef - Clear cell papillary renal cell carcinoma is the fourth most common histologic type of renal cell carcinoma in 290 consecutive nephrectomies for renal cell carcinoma
Haijun Zhou, Shaojiang Zheng, Luan D. Truong, Jae Y. Ro, Alberto G. Ayala, Steven S. Shen
Human Pathology.2014; 45(1): 59. CrossRef - Clear cell papillary renal cell carcinoma: Incidence, morphological features, immunohistochemical profile, and biologic behavior: A single institution study
Borislav A. Alexiev, Cinthia B. Drachenberg
Pathology - Research and Practice.2014; 210(4): 234. CrossRef - MRI Phenotype in Renal Cancer
Naomi Campbell, Andrew B. Rosenkrantz, Ivan Pedrosa
Topics in Magnetic Resonance Imaging.2014; 23(2): 95. CrossRef
- Vascular, adipose tissue, and/or calyceal invasion in clear cell tubulopapillary renal cell tumour: potentially problematic diagnostic scenarios
- Multifocal Renal Cell Carcinoma of Different Histological Subtypes in Autosomal Dominant Polycystic Kidney Disease
- Ki Yong Na, Hyun-Soo Kim, Yong-Koo Park, Sung-Goo Chang, Youn Wha Kim
- Korean J Pathol. 2012;46(4):382-386. Published online August 23, 2012
- DOI: https://doi.org/10.4132/KoreanJPathol.2012.46.4.382
- 10,522 View
- 77 Download
- 13 Crossref
-
 Abstract
Abstract
 PDF
PDF Renal cell carcinoma (RCC) in autosomal dominant polycystic kidney (ADPKD) is rare. To date, 54 cases of RCC in ADPKD have been reported. Among these, only 2 cases have different histologic types of RCC. Here we describe a 45-year-old man who received radical nephrectomy for multifocal RCC with synchronous papillary and clear cell histology in ADPKD and chronic renal failure under regular hemodialysis. The case reported herein is another example of the rare pathological finding of RCC arising in a patient with ADPKD.
-
Citations
Citations to this article as recorded by- Autosomal Dominant Polycystic Kidney Disease-Related Multifocal Renal Cell Carcinoma: A Narrative Iconographic Review
Consolato M. Sergi, Luis Guerra, Josef Hager
International Journal of Molecular Sciences.2025; 26(9): 3965. CrossRef - Autosomal Dominant Polycystic Kidney Disease Patients Requiring Nephrectomy: Characteristics and Surgical Considerations
Joel Ern Zher Chan, Kate S. Olakkengil, Shantanu Bhattacharjya, Santosh Antony Olakkengil
ANZ Journal of Surgery.2025; 95(7-8): 1605. CrossRef - Renal Cell Carcinoma in the Background of Autosomal Dominant Polycystic Kidney Disease: Report of Two Cases and Review of Literature
Poorva Vias, Shikha Goyal, Renu Madan, Nandita Kakkar, Ridhi Sood, Kannan Periasamy, Rajender Kumar
Indian Journal of Medical and Paediatric Oncology.2024; 45(02): 188. CrossRef - Detection of two synchronous histologically different renal cell carcinoma subtypes in the same kidney: a case report and review of the literature
Mohamed Sakr, Merhan Badran, Sarah Ahmed Hassan, Mohamed Elsaqa, Mohamed Anwar Elwany, Nevine M. F. El Deeb, Mohamed Sharafeldeen
Journal of Medical Case Reports.2024;[Epub] CrossRef - The Importance of Genetic Testing in the Differential Diagnosis of Atypical TSC2-PKD1 Contiguous Gene Syndrome—Case Series
Petronella Orosz, Zita Kollák, Ákos Pethő, András Fogarasi, György Reusz, Kinga Hadzsiev, Tamás Szabó
Children.2023; 10(3): 420. CrossRef - Autosomal dominant polycystic kidney disease coming up with an unusual presentation of renal cell carcinoma on its first encounter
Asma Shoukat Masumdar, Anitha Padmanabhan, Nitin Gadgil, Gargi Padalkar
Indian Journal of Pathology and Oncology.2023; 10(4): 417. CrossRef - Sarcomatoid renal cell carcinoma with autosomal dominant polycystic kidney disease: a case report and literature review
Yuji Hakozaki, Kiyotaka Uchiyama, Akane Yanai, Daisuke Yamada, Yuka Kamijo, Yoshitaka Ishibashi
CEN Case Reports.2021; 10(2): 199. CrossRef - CT and MRI findings of cystic renal cell carcinoma: comparison with cystic collecting duct carcinoma
Qingqiang Zhu, Jun Ling, Jing Ye, Wenrong Zhu, Jingtao Wu, Wenxin Chen
Cancer Imaging.2021;[Epub] CrossRef - Incidental occurrence of papillary renal cell carcinoma in the native kidney with autosomal dominant polycystic kidney disease after renal transplantation: A case report
Mahmoud Abbas, Melanie Pätzel, Angelika Thurn, Olaf Brinkmann, Olaf Bettendorf
Molecular and Clinical Oncology.2021;[Epub] CrossRef - Xp11.2 translocation renal cell carcinoma in the autosomal dominant polycystic kidney disease patient with preserved renal function
Hyuk Huh, Hyung Ah Jo, YongJin Yi, Seung Hyup Kim, Kyung Chul Moon, Curie Ahn, Hayne Cho Park
The Korean Journal of Internal Medicine.2017; 32(6): 1108. CrossRef - The Association between Autosomal Dominant Polycystic Kidney Disease and Renal Cell Carcinoma
Chase C. Hansen, Michael Derrick, Irfan Warriach, James Thomas Cammack, James Thomas Cammack, Werner de Riese
Open Journal of Urology.2015; 05(06): 84. CrossRef - The MSCT and MRI findings of collecting duct carcinoma
Q. Zhu, J. Wu, Z. Wang, W. Zhu, W. Chen, S. Wang
Clinical Radiology.2013; 68(10): 1002. CrossRef - Thyroid-like follicular carcinoma of the kidney in a patient with nephrolithiasis and polycystic kidney disease: a case report
Metka Volavšek, Margareta Strojan-Fležar, Gregor Mikuz
Diagnostic Pathology.2013;[Epub] CrossRef
- Autosomal Dominant Polycystic Kidney Disease-Related Multifocal Renal Cell Carcinoma: A Narrative Iconographic Review
- Cyclooxygenase-2 Expression and Its Prognostic Significance in Clear Cell Renal Cell Carcinoma
- Ji Won Lee, Jeong Hwan Park, Ja Hee Suh, Kyung Han Nam, Ji-Young Choe, Hae Yoen Jung, Ji Yoen Chae, Kyung Chul Moon
- Korean J Pathol. 2012;46(3):237-245. Published online June 22, 2012
- DOI: https://doi.org/10.4132/KoreanJPathol.2012.46.3.237
- 9,931 View
- 52 Download
- 16 Crossref
-
 Abstract
Abstract
 PDF
PDF Background The prognostic value of cyclooxygenase-2 (COX-2) in human renal cell carcinoma (RCC) remains unclear. The purposes of this study are to elucidate the clinical significance of COX-2 in clear cell RCC (CCRCC) and to assess the treatment effect of COX-2 inhibition on CCRCC cell lines.
Methods Using tumor samples obtained from 137 patients who had undergone nephrectomy at Seoul National University Hospital, we evaluated COX-2 expression on immunohistochemistry. Moreover, we performed the cell proliferation assay using 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl-2H tetrazolium bromide (MTT) and cell invasion assay. Thus, we evaluated the effect of meloxicam, an inhibitor of COX-2, in two human CCRCC cell lines.
Results Cancer-specific survival (p=0.038) and progression-free survival (p=0.031) were shorter in the COX-2 high expression group. A multivariate logistic regression model showed that COX-2 expression was an independent risk factor for pTNM stage and Fuhrman nuclear grade. The MTT assay revealed that COX-2 inhibition led to the suppression of the proliferation of CCRCC cell lines. Moreover, it also reduced their invasion capacity.
Conclusions This study postulates that COX-2 is a poor prognostic indicator in human CCRCC, suggesting that COX-2 inhibition can be a potential therapy in CCRCC.
-
Citations
Citations to this article as recorded by- Arachidonic acid metabolism as a therapeutic target in AKI-to-CKD transition
Xiao-Jun Li, Ping Suo, Yan-Ni Wang, Liang Zou, Xiao-Li Nie, Ying-Yong Zhao, Hua Miao
Frontiers in Pharmacology.2024;[Epub] CrossRef - The tumor microenvironment and immune targeting therapy in pediatric renal tumors
Amy B. Hont, Benoit Dumont, Kathryn S. Sutton, John Anderson, Alex Kentsis, Jarno Drost, Andrew L. Hong, Arnauld Verschuur
Pediatric Blood & Cancer.2023;[Epub] CrossRef - Free-fatty acid receptor-1 (FFA1/GPR40) promotes papillary RCC proliferation and tumor growth via Src/PI3K/AKT/NF-κB but suppresses migration by inhibition of EGFR, ERK1/2, STAT3 and EMT
Priyanka F. Karmokar, Nader H. Moniri
Cancer Cell International.2023;[Epub] CrossRef - Flavonoids derived from Anemarrhenae Rhizoma ameliorate inflammation of benign prostatic hyperplasia via modulating COX/LOX pathways
Xiaotong Cao, Ying Shang, Weigui Kong, Shuqing Jiang, Jun Liao, Ronghua Dai
Journal of Ethnopharmacology.2022; 284: 114740. CrossRef - Kirenol, darutoside and hesperidin contribute to the anti-inflammatory and analgesic activities of Siegesbeckia pubescens makino by inhibiting COX-2 expression and inflammatory cell infiltration
Yu-Sang Li, Jian Zhang, Gui-Hua Tian, Hong-Cai Shang, He-Bin Tang
Journal of Ethnopharmacology.2021; 268: 113547. CrossRef - Differential expression of cyclooxygenase-2 and cyclin D1 in salivary gland tumors
Jefferson da Rocha Tenório, Leorik Pereira da Silva, Marília Gabriela de Aguiar Xavier, Thalita Santana, George João Ferreira do Nascimento, Ana Paula Veras Sobral
European Archives of Oto-Rhino-Laryngology.2018; 275(9): 2341. CrossRef - Retrospective evaluation ofCOX‐2 expression, histological and clinical factors as prognostic indicators in dogs with renal cell carcinomas undergoing nephrectomy
S. Carvalho, A. L. Stoll, S. L. Priestnall, A. Suarez‐Bonnet, K. Rassnick, S. Lynch, I. Schoepper, G. Romanelli, P. Buracco, M. Atherton, E. M. de Merlo, A. Lara‐Garcia
Veterinary and Comparative Oncology.2017; 15(4): 1280. CrossRef - Functional PTGS2 polymorphism-based models as novel predictive markers in metastatic renal cell carcinoma patients receiving first-line sunitinib
Arancha Cebrián, Teresa Gómez del Pulgar, María José Méndez-Vidal, María Luisa Gonzálvez, Nuria Lainez, Daniel Castellano, Iciar García-Carbonero, Emilio Esteban, Maria Isabel Sáez, Rosa Villatoro, Cristina Suárez, Alfredo Carrato, Javier Munárriz-Ferránd
Scientific Reports.2017;[Epub] CrossRef - COX-2 expression in ovarian cancer: an updated meta-analysis
Haiming Sun, Xuelong Zhang, Donglin Sun, Xueyuan Jia, Lidan Xu, Yuandong Qiao, Yan Jin
Oncotarget.2017; 8(50): 88152. CrossRef - COX-2 Expression in Renal Cell Carcinoma and Correlations with Tumor Grade, Stage and Patient Prognosis
Hedieh Moradi Tabriz, Marzieh Mirzaalizadeh, Shahram Gooran, Farzaneh Niki, Maryam Jabri
Asian Pacific Journal of Cancer Prevention.2016; 17(2): 535. CrossRef - Lipidomic Signatures and Associated Transcriptomic Profiles of Clear Cell Renal Cell Carcinoma
Kosuke Saito, Eri Arai, Keiko Maekawa, Masaki Ishikawa, Hiroyuki Fujimoto, Ryo Taguchi, Kenji Matsumoto, Yae Kanai, Yoshiro Saito
Scientific Reports.2016;[Epub] CrossRef - Intratumoral expression of cyclooxygenase-2 (COX-2) is a negative prognostic marker for patients with cutaneous melanoma
Łukasz Kuźbicki, Dariusz Lange, Agata Stanek-Widera, Barbara W. Chwirot
Melanoma Research.2016; 26(5): 448. CrossRef - New Insights on COX-2 in Chronic Inflammation Driving Breast Cancer Growth and Metastasis
Honor J. Hugo, C. Saunders, R. G. Ramsay, E. W. Thompson
Journal of Mammary Gland Biology and Neoplasia.2015; 20(3-4): 109. CrossRef - The Role of Prostaglandin E2 in Renal Cell Cancer Development: Future Implications for Prognosis and Therapy
Katarzyna Kaminska, Cezary Szczylik, Fei Lian, Anna M Czarnecka
Future Oncology.2014; 10(14): 2177. CrossRef - Genomics and epigenomics of clear cell renal cell carcinoma: Recent developments and potential applications
Małgorzata Rydzanicz, Tomasz Wrzesiński, Hans A.R. Bluyssen, Joanna Wesoły
Cancer Letters.2013; 341(2): 111. CrossRef - Quantitative Assessment of the Association of COX-2 (Cyclooxygenase-2) Immunoexpression with Prognosis in Human Osteosarcoma: A Meta-Analysis
Zhe Wang, Maolin He, Zengming Xiao, Hao Wu, Yang Wu, Dominique Heymann
PLoS ONE.2013; 8(12): e82907. CrossRef
- Arachidonic acid metabolism as a therapeutic target in AKI-to-CKD transition
- Prognostic Significance and Nature of Rhabdoid Features in Renal Cell Carcinoma.
- Misun Choe, Ji Young Park, Ilseon Hwang, Sang Pyo Kim
- Korean J Pathol. 2011;45(4):371-378.
- DOI: https://doi.org/10.4132/KoreanJPathol.2011.45.4.371
- 4,751 View
- 38 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Recent reports have indicated that renal cell carcinoma (RCC) with rhabdoid features follows an aggressive clinical course. We investigated the prognostic significance and nature of the rhabdoid component.
METHODS
We retrospectively analyzed the incidence and clinicopathologic characteristics of RCC with rhabdoid features in 174 radical nephrectomy cases. The specimens were examined histologically and immunohistochemically.
RESULTS
Twelve of the 174 RCC cases (6.9%) showed rhabdoid features. Histologically, all the tumors with rhabdoid features were of the clear cell type. The presence of rhabdoid features was significantly associated with higher Fuhrman's nuclear grade and higher pathologic tumor stage at presentation. Among the 12 patients who showed the rhabdoid component, nine (75%) developed metastasis and seven (58.3%) died of disease-related causes. The presence of rhabdoid features was independently associated with metastasis and disease-related mortality. The rhabdoid cells were positive for vimentin; variably positive for pan-cytokeratin, epithelial membrane antigen, and CD10; and negative for cytokeratin 7, smooth muscle actin, desmin, E-cadherin, and c-Kit. No case showed loss of integrase interactor-1; one was p53 positive, and five were insulin-like growth factor mRNA binding protein 3 positive. The Ki-67 labeling index was 1-25% (mean, 5.5%).
CONCLUSIONS
The rhabdoid component is an independent prognostic factor for metastasis of RCC; therefore, identification of this component is critical.
- Mucinous Tubular and Spindle Cell Carcinoma of the Kidney with Aggressive Behavior: An Unusual Renal Epithelial Neoplasm: A Case Report.
- Ji Hye Lee, Mee Hye Oh, Hyun Deuk Cho, Young Sik Kim
- Korean J Pathol. 2010;44(2):211-215.
- DOI: https://doi.org/10.4132/KoreanJPathol.2010.44.2.211
- 4,461 View
- 31 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF - Mucinous tubular and spindle cell carcinoma is a rare low-grade renal cell carcinoma, which was first described as a new entity in the World Health Organization 2004 classification. We report here on a case of this tumor with very unusual aggressive behavior. A 73-year-old man presented with gross hematuria. A computed tomography scan demonstrated a 5 cm sized low density mass in the left kidney. The radical nephrectomy specimen grossly showed a well demarcated tumor confined to the renal parenchyma. Histologically, the tumor consisted of elongated tubules or trabeculae of oval to cuboidal cells with a low nuclear grade, and these tubules/trabeculae were separated by abundant acidic mucinous stroma. In some areas, spindle cell components were mixed with parallel tubules. Neither significant atypia nor mitosis was seen. The patient developed multiple metastatic pulmonary nodules 2 months later. Four months after the surgery, the left supraclavicular, right hilar and right subcarinal lymph nodes were also enlarged by metastasis. The patient died of respiratory failure 13 months after the operation.
-
Citations
Citations to this article as recorded by- Mucinous spindle and tubular renal cell carcinoma: analysis of chromosomal aberration pattern of low-grade, high-grade, and overlapping morphologic variant with papillary renal cell carcinoma
Kvetoslava Peckova, Petr Martinek, Maris Sperga, Delia Perez Montiel, Ondrej Daum, Pavla Rotterova, Kristýna Kalusová, Milan Hora, Kristýna Pivovarcikova, Boris Rychly, Semir Vranic, Whitney Davidson, Josef Vodicka, Magdaléna Dubová, Michal Michal, Ondrej
Annals of Diagnostic Pathology.2015; 19(4): 226. CrossRef
- Mucinous spindle and tubular renal cell carcinoma: analysis of chromosomal aberration pattern of low-grade, high-grade, and overlapping morphologic variant with papillary renal cell carcinoma
- Diagnostic Utility of AMACR and Claudin-7 for the Classification of Renal Cell Carcinoma.
- Sang Hwa Shim, Mee Joo, Han Seong Kim, Sun Hee Chang, Ki Young Kwon
- Korean J Pathol. 2010;44(2):155-161.
- DOI: https://doi.org/10.4132/KoreanJPathol.2010.44.2.155
- 4,146 View
- 53 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
The histologic classification of renal cell carcinoma (RCC) is based on the cytoarchitectural features, yet sometimes this requires correlation with the immunophenotype. Alpha-methylacyl-CoA racemase (AMACR) and claudin-7 have recently been introduced as useful markers that are frequently expressed in papillary RCC (PRCC) and chromophobe RCC (ChRCC), respectively. The aims of this study are to evaluate the expressions of AMACR and claudin-7 in RCCs and to investigate whether they are helpful for making the histological classification of RCCs.
METHODS
Immunohistochemistry for CD10, RCC marker, cytokeratin (CK)7, CD117, AMACR and claudin-7 was performed for 104 RCCs, and these consisted of 54 clear cell RCCs (CCRCC), 26 PRCCs and 24 ChRCCs.
RESULTS
For diagnosing PRCC, the sensitivity and specificity of AMACR were 92.3% and 71.8%, respectively, and using AMACR(+)/CK7(+), the specificity was increased by 23.1% to 94.9%. For diagnosing ChRCC, the sensitivity and specificity of claudin-7 were 91.7% and 78.8%, respectively, and using claudin-7(+)/AMACR(-), the specificity was significantly improved (to 96.3%). For diagnosing CCRCC, CK7(-)/claudin-7(-)/CD117(-) was the most useful immunohistochemical panel (sensitivity, 96.3%; specificity, 98%).
CONCLUSIONS
AMACR and claudin-7 are helpful markers for the histologic classification of RCCs, and their diagnostic utility is strengthened when they are used as an immunohistochemical panel, AMACR(+)/CK7(+) for PRCC, claudin-7(+)/AMACR(-) for ChRCC and CK7(-)/claudin-7(-)/CD117(-) for CCRCC.
- The Prognostic Implications of Cystic Change in Clear Cell Renal Cell Carcinoma.
- Heae Surng Park, Eun Jung Jung, Jae Kyung Myung, Kyung Chul Moon
- Korean J Pathol. 2010;44(2):149-154.
- DOI: https://doi.org/10.4132/KoreanJPathol.2010.44.2.149
- 6,376 View
- 94 Download
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Cystic renal cell carcinoma has been reported to have a good prognosis. However, previous studies included cases of multilocular cystic renal cell carcinoma, which has an excellent prognosis, and renal cell carcinoma with cystic necrosis, which has an adverse prognosis. Therefore, we analyzed the prognostic influence of cystic change in clear cell renal cell carcinoma after excluding those morphological features.
METHODS
We identified 225 patients with clear cell renal cell carcinoma who underwent nephrectomy between 2001 and 2003. The clinicopathologic features were compared with clinical outcomes.
RESULTS
Cystic change in clear cell renal cell carcinoma (n = 66) was significantly associated with younger patient age (< 55), smaller tumor size (< or = 4 cm), lower pT stage (pT1, T2), M0 stage at initial diagnosis, lower tumor, node, and metastasis stage (I, II), and lower nuclear grade (1, 2). Patients with cystic change in clear cell renal cell carcinoma had significantly longer cancer-specific (p = 0.015) and progression-free survival (p = 0.004) than those without cystic change, by univariate analysis. Multivariate analysis revealed that cystic change significantly decreased the risk of cancer progression (risk ratio, 0.27; 95% confidence interval, 0.11 to 0.69).
CONCLUSIONS
In patients with clear cell renal cell carcinoma, cystic change is a good independent predictor for survival. -
Citations
Citations to this article as recorded by- Update on MRI of Cystic Renal Masses Including Bosniak Version 2019
Satheesh Krishna, Nicola Schieda, Ivan Pedrosa, Nicole Hindman, Ronaldo H. Baroni, Stuart G. Silverman, Matthew S. Davenport
Journal of Magnetic Resonance Imaging.2021; 54(2): 341. CrossRef - Klotho plays a critical role in clear cell renal cell carcinoma progression and clinical outcome
Ji-Hee Kim, Kyu-Hee Hwang, Sayamaa Lkhagvadorj, Jae Hung Jung, Hyun Chul Chung, Kyu-Sang Park, In Deok Kong, Minseob Eom, Seung-Kuy Cha
The Korean Journal of Physiology & Pharmacology.2016; 20(3): 297. CrossRef - Insulin Receptor Expression in Clear Cell Renal Cell Carcinoma and Its Relation to Prognosis
Sayamaa Lkhagvadorj, Sung Soo Oh, Mi-Ra Lee, Jae Hung Jung, Hyun Chul Chung, Seung-Kuy Cha, Minseob Eom
Yonsei Medical Journal.2014; 55(4): 861. CrossRef - Determination of the Cutoff Value of the Proportion of Cystic Change for Prognostic Stratification of Clear Cell Renal Cell Carcinoma
Heae Surng Park, Kyoungbun Lee, Kyung Chul Moon
Journal of Urology.2011; 186(2): 423. CrossRef
- Update on MRI of Cystic Renal Masses Including Bosniak Version 2019
- The EGFR Protein Expression and the Gene Copy Number Changes in Renal Cell Carcinomas.
- Sangho Lee, Jungsuk An, Aeree Kim, Young Sik Kim, Insun Kim
- Korean J Pathol. 2009;43(5):413-419.
- DOI: https://doi.org/10.4132/KoreanJPathol.2009.43.5.413
- 4,613 View
- 21 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
The epidermal growth factor receptor (EGFR) is known to be involved in many tumor promoting activities. EGFR inhibition has been tried as a therapeutic modality in many human malignancies.
METHODS
The expression of EGFR protein and the gene copy number changes were studied in 135 clear cell carcinomas and 16 papillary renal cell carcinomas (RCCs), and these tumors were diagnosed between 1995 and 1997.
RESULTS
An EGFR protein expression (2+ and 3+) was found in 54.1% of the clear cell RCCs and in 43.8% of the papillary RCCs. In the clear cell RCCs, its expression was associated with male gender, the tumor size (> or =4 cm) and high T stages (T2 and T3), with statistical significance. Trisomy and polysomy of the EGFR gene were found in 27 (25.7%) and 40 (38.1%) of 105 clear cell RCCs, respectively. Trisomy and polysomy were correlated with an EGFR protein expression and a high clinical T stage, with statistical significance. Among 15 papillary RCCs, 13 tumors showed trisomy (86.7%) and one showed polysomy (6.7%). Amplification was not found in both the clear cell and papillary type RCCs.
CONCLUSIONS
A considerable numbers of RCCs showed an overexpression of EGFR protein and increased EGFR gene copy numbers, yet the clinical significance of conducting a FISH study in RCC patients seems to be limited. -
Citations
Citations to this article as recorded by- EGFR protein overexpression correlates with chromosome 7 polysomy and poor prognostic parameters in clear cell renal cell carcinoma
Gordana Đorđević, Koviljka Matušan Ilijaš, Ita Hadžisejdić, Anton Maričić, Blaženka Grahovac, Nives Jonjić
Journal of Biomedical Science.2012;[Epub] CrossRef
- EGFR protein overexpression correlates with chromosome 7 polysomy and poor prognostic parameters in clear cell renal cell carcinoma
- The Relationship between Prognostic Factors and the Expression Pattern of Fascin and E-cadherin in Renal Cell Carcinoma.
- Sung Hee Kang, Seoung Wan Chae, Kyoung Bun Lee, Dong Hoon Kim, Min Kyoung Kim, Jin Hee Sohn
- Korean J Pathol. 2009;43(2):139-144.
- DOI: https://doi.org/10.4132/KoreanJPathol.2009.43.2.139
- 3,475 View
- 19 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Fascin is associated with motility in various transformed cells. Overexpression of fascin is known to aid in the progression of some cancers and is associated with a poor prognosis. E-cadherin is a major protein of epithelial cells and its expression is involved in the regulation of cell proliferation and differentiation. The aim of this study was to determine the expression pattern for fascin and E-cadherin and how it is related to the prognostic factors for renal cell carcinoma (RCC).
METHODS
The expression of fascin and E-cadherin was evaluated in 208 RCCs including 175 clear cell, 20 papillary, and 9 chromophobe types using tissue array analysis.
RESULTS
The expression of fascin increased as the tumor stage (p=0.00) and Fuhrman grade (p=0.00) increased. A high positive rate of expression for fascin was observed in cases with sarcomatoid changes (p=0.27). E-cadherin expression was seen in the distal tubules and collecting ducts of normal kidneys with a membranous pattern. The positive rate of expression for E-cadherin increased as the Fuhrman grade increased (1, 0%; 2, 23.2%; 3, 34.9%; and 4, 53.8%, p=0.00). An inverse correlation in RCCs was observed in the expression of fascin and E-cadherin (p=0.026, r=-0.158).
CONCLUSIONS
In patients with RCC, the increased expression of fascin and E-cadherin was positively correlated to poor prognostic factors such as a higher Fuhrman nuclear grade and advanced pTNM stage.
- A Pathologic Study of Renal Cell Carcinoma: Correlation between clinical and morphologic parameters and prognosis.
- Hye Seon Ahn, Moon Hyang Park
- Korean J Pathol. 1992;26(6):561-572.
- 1,843 View
- 12 Download
-
 Abstract
Abstract
 PDF
PDF - The prognostic significance of morphologic parameters was evaluated in 36 cases of renal cell carcinoma diagnosed during five years(1986~1990). We reviewed and classified on the basis of pathologic stage, tumor size, histologic pattern, cell type and nuclear grade. Mean age was 51 years old. Average tumor size was 7.3 cm in diameter. Six of 35 patients died of disease. Overall mean survival was 43.3+/-7.3 months. An increasing nuclear grade was generally correlated with a decrease in cummlative survival rate. Similarly, a higher stage at the time of diagnosis could predicated a low survival rate only for high nuclear grade carcinoma. There was an apparent positive correlation between grade and age, grade and size, grade and cell type, cell type and histologic pattern as well as stage and age. This positive correlations are in part a function of nuclear grade; only 20% of grade 3 & 4 tumor consisted of clear cells whereass 71% of grade 1 & 2 consisted of clear cell type. All 6 cases of granular cell types and 50% of mixed cell type were grade 3 & 4. The tumor size of the primary was well correlated with the nuclear grade. Nuclear grade was the most significant factor among the morphologic parameters studied.
- A Pathological Study of Renal Cell Carcinoma.
- Kwang Hwa Park, Dong Hwan Shin, In Joon Choi
- Korean J Pathol. 1989;23(3):322-330.
- 2,170 View
- 16 Download
-
 Abstract
Abstract
 PDF
PDF - The most common malignant renal neoplasm is renal cell carcinoma. It is estimated that renal cell carcinoma accounts for 1% of all primary malignancies in Korea. Rell cell carcinoma presents diverse clinical courses with gross, histopathologic features. It has been known to be very difficult tumor to predict its clinical prognosis. In Korea, many studies have been reported concerning the clinical aspects of renal cell carcinoma. However, pathological studies of renal cell carcinoma are very few even though studies of nuclear grade have been attempted recently. We reviewed 93 cases of renal cell carcinoma examined in the period from 1978 to 1987 in the department of pathology, Yonsei university college of medicine, Yongdong Severance hospital, Wonju college of medicine and analyzed the histopathologic classification, including nuclear grade according to the Fuhrman's method. We abtained the following results by studying the relationship of the factors which had been known as correlated with the prognosis. 1) The ages of patients ranged from 9 to 74 years with a peak in the 6th decade. 2) The most common symptoms of the patients were hematuria, mass and pain, in that oder, and 7 patients complained to specific symptoms. The incidentally found cases characterized stage I, nuclear grade 2 small tumor size (not more than 4 cm) and clear cell type. 3) The renal cell carcinoma was more frequently located in the left kidney than the right by a ratio of 1.25 : 1. The incidence of intrarenal location was divided to the upper pole, 40% : mid portion, 29% : lower pole, 23% : diffuse involvement, 8%. The tumor shoing diffuse growth pattern had a large size, high nuclear grade and mixed cells. 4) The tumor size averaged 8 cm and there was no significant relationship between the size and stage. Seven cases of neoplasms not more than 3 cm were seen, of which 2 cases revealed an outcome of distant metastasis. 5) The histological pattern showed major solid, 53% : tubular, 11% : mixed, 18% : papillary, 9% and sarcomatoid type 9%. The sarcomatoid type was characterized by grade 4, a larger size(more than 10 cm), advanced stage. 6) There was no special relationship between the stage and grade but mostly grade 2 occupied the stage I. 7) The clear cell type was predominantly noted at grade 2 (65%), at the stage I (63%), granular or mixed cell type at grade 3 (87%), 4 (70%). According to these results, the tumors showing a sarcomatoid histologic pattern, diffuse growth pattern had unfavorable prognostic factors, and are thus estimated to have a poor prognosis. But the case which were incidentally found have favorable prognostic factors and probably a better prognosis. The tumor size alone can not exactly predict the metastasis and is not correlated with the stage. Small renal cell neoplasm (not more than 3 cm) generally has unfavorable prognostic factors and should be considered potentially malignant. The high grade frequently has granular cytoplasm. This represents the relationship between grade and cytoplasm, poor prognosis in the granular cell than the clear. The renal cell carcinoma shows variable prognosis and thus the prognosis should be estimated by all the factors. Nuclear grade can be used as one of the useful prognostic factors.
- Chromophobe Renal Cell Carcinoma.
- Yeong Jin Choi, Tae Kon Hwang, Youn Soo Lee, Eun Jung Lee, Seok Jin Kang, Byung Kee Kim, Sang In Shim
- Korean J Pathol. 1999;33(4):259-266.
- 2,186 View
- 26 Download
-
 Abstract
Abstract
 PDF
PDF - We report 13 chromophobe renal cell carcinomas (10.8%) observed among 120 renal cell carcinomas in adults. The average age was 53 (range: 34-72) years old, and 6 were males and 7 females. The mean tumor size was 10 (range: 5-17) cm, mean nuclear grade 2.4, and mean Robson's stage was 1.9. There were two distinct histologic variants; typical variant (n=9) and eosinophilic variant (n=4). Both of them showed typical light microscopic features and positive reaction with Hale's colloidal iron and carbonic anhydrase II, a marker protein of intercalated cells of renal collecting ducts. A strong positive immunoreactivity for epithelial membrane antigen was noted in the cytoplasm in 12 of 13 tumors. Numerous microvesicles, 180~440 nm in diameter, were identified ultrastructurally. DNA aneuploidy was found in 3 out of 10 cases. Neither local recurrence nor metastasis have been identified during the following period of 4~144 (mean 48) months.
- Immunohistochemical Markers for Metastasis in Clear Cell Renal Cell Carcinoma.
- Kyungeun Kim, Cheryn Song, Jae Y Ro, Hanjong Ahn, Yong Mee Cho
- Korean J Pathol. 2008;42(2):81-86.
- 2,488 View
- 25 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Renal cell carcinoma (RCC) is notorious for its high metastatic potential, and 30% of RCC patients present with metastatic disease at the initial presentation and 50% of them will develop metastasis or recurrence after radical surgery.
METHODS
In an attempt to identify the best predictive marker(s) for metastasis in patients with clear cell RCCs (CRCCs), we examined the expression patterns of 7 metastasis/prognosis-related markers by constructing a tissue microarray including primary CRCC specimens from 30 metastatic and 60 nonmetastatic CRCCs. The markers we studied were Ki-67, MUC1, CD44s, PTEN, gelsolin, CA9 and p53.
RESULTS
The expressions of Ki-67, PTEN, CD44s, gelsolin and p53 were increased, whereas those of MUC1 and CA9 were decreased in the metastatic CRCCs compared with the non-metastatic CRCCs. The receiver operating characteristic curve-area under the curve (AUC) value of Ki-67 was 0.671, which was the highest among the 7 markers. The optimal cut-off value, sensitivity and specificity of the Ki-67 expression were 1.67%, 86.7% and 41.7%, respectively.
CONCLUSIONS
These results demonstrate that the Ki-67 expression was increased in metastatic CRCCs, and it had the highest predictive value among the 7 markers. This suggests that Ki-67 could be an excellent predictive marker for metastasis in CRCC patients.
- A Study on the Expression of p53 Oncogene Products, PCNA Index and DNA Ploidy in Renal Cell Carcinoma.
- Jong Jae Jung, Ji Shin Lee, Chan Choi
- Korean J Pathol. 1997;31(7):672-682.
- 1,937 View
- 20 Download
-
 Abstract
Abstract
 PDF
PDF - Mutant p53 is associated with the advanced stages of some human tumor but there is a wide variation in the reported incidence of p53 mutation in renal cell carcinoma and its prognostic significances. We designed this study to assess the expression of p53 in renal cell carcinomas and to compare with the established prognostic factors. Immunoreactivity for p53 protein and proliferating cell nuclear antigen (PCNA) were assessed in 44 cases of primary renal cell carcinoma, and flow cytometric analysis of DNA ploidy was perfon-ned in 37 of those cases. p53 protein was over-expressed in 16/44 (36.4%) renal cell carcinomas and 5 rumors had more than 10 immunoreactive tumor cells. The expression of p53 protein was positively related to nuclear grade (p=0.007) and PCNA index (p=0.002), but was independent of stage and DNA ploidy. In univariate survival analysis, stage (p<0.001), nuclear grade (p=0.017), DNA ploidy (p=0.045) and PCNA index (p<0.001) were significantly associated with patient survival. However, considering the stage, all of the last three factors had no prognostic influence. Cases showing strong positivity of p53 expression had worse prognosis than those with no or weak p53 expression, especially in early lesions (stage I,II) (p<0.001).
- A Case Report of Renal Cell Carcinoma in a Polycystic Kidney: A case report.
- Kyoung Chan Choi, Joon Hyuk Choi, Won Hee Choi
- Korean J Pathol. 1996;30(1):57-60.
- 2,031 View
- 18 Download
-
 Abstract
Abstract
 PDF
PDF - A forty-nine-year-old woman with polycystic disease had a right nephrectomy for what was preoperatively thought to be a polycystic disease, but at surgery turned out to be a tumor based on frozen section. Microscopic examination revealed papillary type, renal cell carcinoma with classical features of adult polycystic kidneys. Radiologic findings revealed multiple cysts in the liver. The clinical recognition of a carcinoma developing in polycystic kidneys is often difficult because of the presence of preexisting large renal masses and occasional hematuria. Renal cell carcinoma should be thought of when confronted with abdominal pain or back pain, severe hematuria, sudden dysuria or a new renal mass occurring in a patient with polycystic kidneys.
- Triple Synchronous Cancers of Stomach, Pancreas, and Kidney.
- Seung Koo Lee, Byung Ha Choi, Shin Kwang Khang, Byung Sik Kim, Jooryung Huh
- Korean J Pathol. 2001;35(6):547-550.
- 2,057 View
- 10 Download
-
 Abstract
Abstract
- Synchronous occurrence of triple distinct malignant tumors in the same patient is very rare. We report a unique case of a triple cancer occurring in a 70-year-old Korean woman with synchronous signet ring cell carcinoma of the stomach, renal cell carcinoma of the conventional type of the left kidney, and invasive ductal adenocarcinoma and intraductal papillary carcinoma of the pancreas. All three cancers were successfully resected simultaneously by total gastrectomy, nephrectomy, and partial pancreatectomy with corresponding lymphadenectomies. This patient tolerated these surgical procedures well and led a normal healthy life during the 18 months of follow-up. In summary, a successful resection of synchronous triple cancers which has never been previously reported in such combination, is described.
- The Prognostic Implications of the Histologic Subtype and the Expression of Phosphorylated ERK 1/2 in Papillary Renal Cell Carcinoma.
- Bo Sung Kim, Dong Il Kim, Tae Hoon Kang, Eun Shin, Kyung Chul Moon
- Korean J Pathol. 2008;42(4):215-222.
- 2,037 View
- 16 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
The authors of this study wanted to confirm the prognostic implication of the histologic subtype; further, we wanted to explore the expression of phosphorylated extracellular signal-regulated kinase 1/2 (pERK) in papillary renal cell carcinoma (PRCC) and determine its clinicopathologic and prognostic significance.
METHODS
A total of 45 patients who underwent nephrectomy for PRCC were enrolled in this study. The hematoxylin and eosin slides were reviewed and pERK immunohistochemistry was performed.
RESULTS
Type 2 PRCC was significantly correlated with a larger tumor size (p=0.030), a higher nuclear grade (p<0.001), a more advanced tumor stage (p=0.041) and more frequent distant metastasis (p=0.019). The tumors were pERK-low (0 and 1+) in 30 cases (66.7%) and pERK-high (2+) in 15 cases (33.3%). The pERK-high PRCC was significantly associated with a smaller tumor size (p=0.001) and an earlier tumor stage (p=0.004). On the univariate analysis, the histologic subtype, the TNM stage and the pERK status were significantly associated with progression-free survival (PFS). Multivariate analysis showed that the histologic subtype (hazard ratio 22.81, p=0.042) and the TNM stage (hazard ratio 23.48, p=0.009) were independent prognostic factors for PFS.
CONCLUSIONS
Type 2 PRCC, together with the TNM stage, was identified as one of independent poor prognostic factors for PFS. The pERK status was a prognostic factor for PFS on the univariate analysis, but not on the multivariate analysis.
- Correlation between Nuclear Grades and the Numbers of AgNORs and PCNA Labeling Indices in Renal Cell Carcinoma.
- Hye Jin Lee, Young Im Han, Kang Suek Suh, Sun Kyung Lee
- Korean J Pathol. 1996;30(2):132-139.
- 1,813 View
- 12 Download
-
 Abstract
Abstract
 PDF
PDF - The author examined the number of AgNORs and PCNA labeling indices by histochemical and immunohistochemical studies in 20 cases of renal cell carcinoma, composed of 5 cases according to the nuclear grades. The results obtained are summarized as follows; 1) Mean number of AgNORs according to the nuclear grades of renal cell carcinoma were 1.38+/-0.40 (mean+/-standard deviation) for Grade I, 2.53+/-0.33 for Grade II, 5.43+/-0.66 for Grade III, and 7.88+/-0.72 for Grade IV. The mean numbers of AgNORs according to the nuclear grades were significantly increased(p=0.0005). 2) PCNA labeling indices (positive nuclear ratio) according to the nuclear grades of renal cell carcinoma were 5.90+/-2.36 for Grade I, 19.30+/-6.71 for Grade II, 45.73+/-8.62 for Grade III, and 61.83+/-6.34 for Grade IV. Also, the PCNA labeling indices according to the nuclear grades were significantly increased(p=0.0008). 3) The mean numbers of AgNORs directly correlated with the PCNA labeling indices (r=0.9861, p<0.001). On the basis of the above results, it was considered that the numbers of AgNORs and PCNA labeling indices as markers of proliferative activity of tumor cells correlate well with the nuclear grades of renal cell carcinoma.
- Multilocular Cystic Renal Cell Carcinoma.
- Myoung Jin Ju, Kee Tac Jang, Je Geun Chi
- Korean J Pathol. 1997;31(11):1240-1243.
- 1,924 View
- 10 Download
-
 Abstract
Abstract
- Multilocular cystic renal cell carcinoma is a distinct subtype of renal cell carcinoma with its pathological characteristics and good prognosis. Multilocular renal cysts and renal cell carcinoma with cystic change are important differential diagnoses. We report a case of multilocular cystic renal cell carcinoma in a 37-year-old woman who came to the hospital because of the right renal mass. The removed right kidney showed a 6x4 cm well defined cystic mass in the lower pole. On cut section there were multiple cavities in the mass, filled with serosanguineous fluid and focal yellowish solid area. Microscopically, these cysts were lined by a single layer of flat or cuboidal cells consisted of clear cytoplasm with small central nuclei. In some portions of the tumor, the clear neoplastic cells formed sheets within the septa or walls of the cysts.
- Expression of CD44 Isoforms and Its Significance in Renal Cell Carcinoma.
- Ghil Suk Yoon, Hee Yeon Hong, Tae Sook Kim
- Korean J Pathol. 2005;39(4):251-257.
- 2,124 View
- 30 Download
-
 Abstract
Abstract
 PDF
PDF - Background
: CD44 is a transmembranous glycoprotein that participates in cell-cell and cell-matrix interactions, and it also contributes to cell migration. In vitro studies have suggested that the expression of CD44 isoforms is associated with tumor metastasis. Since it is not clear whether the CD44 isoforms play a role in the tumorigenesis, differentiation, progression or metastasis of renal cell carcinomas (RCCs). Methods : We performed immunohistochemistry with primary antibodies for the standard CD44 (CD44s) and the CD44 variant exon 6 (CD44v6) on the archival paraffin-embedded tissue microarray (TMA) specimens from 51 RCC patients. Results : In the normal kidney, the expressions of both CD44s and CD44v6 were negligible. The CD44s expression was increased in accordance with the tumor size (p<0.01), but it was not related to the microvessel density (MVD). No CD44v6 expression was observed in all RCC cases. Univariate analysis indicated that stage, tumor size, lymph node metastasis and distant organ metastasis were the statistically significant prognostic factors for disease free survival (DFS) (p<0.01), and the multivariate analysis proved that stage (p<0.01) and tumor size (p<0.05) were the independent prognostic factors for DFS. Conclusions : Our results suggest that CD44s, but not CD44v6, plays a role in tumor progression and it could be a potential prognostic factor for patients with RCCs.
- Tumor Angiogenesis in Renal Cell Carcinoma.
- Ji Shin Lee, Jong Jae Jung, Chang Soo Park
- Korean J Pathol. 1999;33(11):1055-1060.
- 1,881 View
- 19 Download
-
 Abstract
Abstract
 PDF
PDF - Angiogenesis is essential for the growth of solid tumors. Microvessel counts, which represent a measure of tumor angiogenesis, have been correlated with the overall survival of patients with a variety of malignancies. However, the significance of angiogenesis in renal cell carcinoma remains controversial. To determine whether angiogenesis correlates with prognosis of patients with renal cell carcinoma, we counted the microvessels within the primary tumors and compared their numbers with patients' prognosis. Tumor specimens from 42 patients were investigated. Microvessels were stained with anti-CD34 and anti-factor VIII-related antigen monoclonal antibodies. Significant correlation between microvessel counts for two antibodies was observed (r=0.875, p<0.01), although microvessel counts for CD34 were approximately two times higher. Microvessel counts were higher in clear cell than in non-clear cell carcinoma (p<0.05). These results suggest that immunostaining with anti-CD34 antibody may provide a more sensitive and accurate measure of tumor angiogenesis. There was no correlation between microvessel counts and nuclear grade, or TNM stage. In univariate analyses, nuclear grade and TNM stage were significantly associated with patient survival (p<0.01). But further studies on tumor angiogenesis of renal cell carcinoma are needed before it can be adopted as a prognostic marker.
- Correlation of Clinical Stage and Presumptive Prognostic Factors in Renal Cell Carcinoma.
- Jin Ye Yoo, Hye Jae Cho
- Korean J Pathol. 1999;33(11):1061-1066.
- 1,965 View
- 16 Download
-
 Abstract
Abstract
 PDF
PDF - Renal cell carcinoma is the most common primary cancer of the kidney. The tumor stage is a reliable prognostic marker in renal cell carcinoma which is significantly associated with patient survival. But assessment of other prognostic factors has produced varying and often conflicting results. We reevaluated the significance of varied prognostic parameters in 33 cases of renal cell carcinoma; clinical stage, cell type, histologic pattern, DNA ploidy, Ki-67 labeling index, and bcl-2 oncoprotein expression. We could not statistically prove that DNA ploidy and bcl-2 expression were related to any examined parameters. Cell type was not related to clinical stage nor nuclear grade but there was a significant correlation (p=0.002) between cell type and histologic pattern. Nuclear grade (p=0.007) and Ki-67 labeling index (p=0.036) were significantly related to clinical stage, suggesting their value as complementary prognostic markers for renal cell carcinoma.
- Urine Cytology of Renal Cell Carcinoma: Analysis of 11 cases.
- Yi Kyeong Chun, Hye Jae Cho, Ill Hyang Ko
- J Pathol Transl Med. 1994;5(2):137-142.
- 4,125 View
- 166 Download
-
 Abstract
Abstract
 PDF
PDF - Urine cytology is of limited value in the diagnosis of renal cell carcinoma with reported detection rates of 0~80%. The aim of this study is to demonstrate the usefulness of urine cytology in renal cell carcinoma, In the eleven histologically proven cases of renal cell carcinoma, urinary smears were reevaluated. The cytologic results were as follows; positive for malignant cells in 3 cases (27%), suspicious in 2 cases (18%) and negative in 6 cases (55%). The average diameter of the tumor of the 5 cases reported as positive or suspicious for malignant cells was 9.7cm and 3 had invaded the renal pelvis. The other 6 tumors, reported as negative, were 5.7cm in average diameter and one of them showed involvement of the renal pelvis. These results suggest that urine cytology is considered unsatisfactory in the early detection of renal cell carcinoma. However. careful examination of urinary smear could improye the detection rate especially in more advanced cases involving the renal pelvis as well as those of larger tumors.
- Renal Cell Carcinoma Associated with Rhabdomyosarcomatous Component: Report of a case.
- Mee Soo Chang, Mi Kyung Jee, Kyo Young Lee, Sang In Shim, Sun Moo Kim
- Korean J Pathol. 1987;21(1):40-44.
- 1,980 View
- 13 Download
-
 Abstract
Abstract
 PDF
PDF - Renal cell carcinoma, intimately associated with a sarcomatous component, is a rare malignant renal tumor. There is disagreement whether these represent true sarcomas or are sarcomatoid metaplasia. Therefore, this sarcomatous component is at times still a troublesome problem for pathologists. In March, 1986, we experienced a case of renal cell carcinoma associated with rhabdomyosarcomatous component in 47 year-old woman who had a rapidly enlarged, palpable abdominal mass. Grossly, a spherical renal cell carcinoma, 17x14x10 cm, in upper and middle portions of the right kidney showed extension through the renal capsule into the perirenal fat. Area of myxoid change was evident in the reanl cell carcinoma, with extensive hemorrhagic necrosis. Microscopically, in the myxoid area, there was malignant spindle cell proliferation in which many rhabdomyoblasts showing distinct cross striation could be demonstrated. This rhabdomyosarcomatous component intermixed with renal cell carcinoma of clear cell type could be also identified in the focal area.
- E-Cadherin Expression in Renal Cell Carcinoma according to the Mainz Classification.
- Ju Han Lee, Hyun Deuk Cho, Dale Lee, Nam Hee Won
- Korean J Pathol. 1999;33(12):1131-1138.
- 2,484 View
- 27 Download
-
 Abstract
Abstract
 PDF
PDF - According to the Mainz classification, renal cell carcinoma (RCC) consists of three subtypes: each has characteristic genetic alterations within the chromosomal or mitochondrial DNA. The three subtypes are: clear cell type, chromophil type, and chromophobe type. E-cadherin is a Ca++-dependent adhesion molecule which plays a major role in the maintenance of intercellular adhesion in epithelial tissues. In a normal kidney, E-cadherin is expressed in the distal tubule and the collecting duct, but not in the proximal tubule. We reclassified 110 cases of RCC according to mainz classification. Immunohistochemical staining for E-cadherin was done on twenty eight cases of RCC, including 18 cases of clear cell type, four cases of chromophil type, and six cases of chromophobe type. The results were as follows: 1) of the 110 cases of RCC, 96 cases (87.3%) were of clear cell type, four cases (3.6%) of chromophil type, and ten cases (9.1%) of chromophobe type, 2) there was no significant correlation between the nuclear grade and clinical stage according to each subtype, 3) E-cadherin expression showed a strong positive reaction along the cell membranes in all six cases of chromophobe type. The differential expression of E-cadherin in RCC may suggest that the chromophobe type may have different biologic characteristics from other types of RCC.
- Renal Cell Carcinoma Associated with Xp11.2 Translocation: Clinicopathologic and Immunohistochemical Findings of 4 Cases.
- Sanghui Park, Ji Eun Kwon, Yeon Lim Suh
- Korean J Pathol. 2005;39(6):406-411.
- 2,007 View
- 19 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
The new WHO classification includes the recently described renal cell carcinomas (RCC) that are associated with several different translocations, involving chromosome Xp11.2, and they all result in gene fusions involving the TFE3 gene. The authors describe the clinicopathologic and immunohistochemical findings of 4 patients who had the morphologic features of RCC with Xp11.2 translocations.
METHODS
Among 9 surgically resected and pathologically proven pediatric RCCs, 4 showed a typical RCC histopathology with the Xp11.2 translocation. Immunohistochemical stains were performed for TFE3, AE1/AE3, epithelial membrane antigen, vimentin, HMB45, S-100 protein and CD10.
RESULTS
The 4 study subjects included one male and 3 females, and their chief complaints were gross hematuria and abdominal pain. Histologically, the tumors showed two different histologic types: type 1 tumors (2 cases) that corresponded to those of ASPL-TFE3 RCC, and type 2 tumors (2 cases) that corresponded to PRCC-TFE3 RCC. Nuclear TFE3 immunostaining was seen in 3 cases. All the tumors were immunoreactive for CD10, and vimentin and cytokeratin were expressed in 3 cases and HMB-45 was expressed in 2 cases.
CONCLUSIONS
Our results show that significant numbers of pediatric RCC are translocation-related. Therefore, when one encounters an RCC in the pediatric population, the possibility of a translocation-related RCC should be kept in mind.
- Metastatic Renal Cell Carcinoma in Maxillary Sinus: A case report.
- Gyeong Yeob Gong, Chang Hun Lee, Kang Suek Suh, Sun Kyung Lee
- Korean J Pathol. 1991;25(4):392-394.
- 2,148 View
- 21 Download
-
 Abstract
Abstract
 PDF
PDF - Metastases to the sinonasal tract are infrequent occurrences from primaries below the clavicles. The total number of reported cases to date is less than 100. There is, however, complete unanimity concerning the histologic type of metastatic neoplasm most often encountered. An interesting phenomenon, generally attributable only to breast and renal cell carcinoma, is the late recurrence of the malignant tumor, even 10 or more years after operation. A 61 year-old-male was admitted to ENT due to frequent epistaxis and right facial swelling. CT scan revealed a huge soft tissue density mass I right maxillary sinus with extension into nasopharynx and deviation of nasal septum. The histologic diagnosis was metastatic renal cell carcinoma. He had left nephrectomy because of renal cell carcinoma, 14 years ago. We report a case of metastatic renal cell carcinoma of maxillary sinus in view of rarity, and a brief review of the literature related to this type of tumor is presented.
- Expression of Heat Shock Protein 27 and Apoptosis in Renal Cell Carcinomas.
- Ghil Suk Yoon
- Korean J Pathol. 2006;40(1):39-45.
- 2,136 View
- 23 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Heat shock protein 27 (HSP27) is induced by heat shock and other pathophysiologic stresses, including neoplastic transformation. We examined the relationship between the HSP27 expression and the clinical and histologic parameters to elucidate the biologic and prognostic significance of HSP27 in renal cell carcinomas (RCCs). Its regulation of apoptosis in RCC development was also observed.
METHODS
We performed immunohistochemical studies for HSP27, caspase 3 and TUNEL on paraffin-embedded tissue microarray specimens from 48 RCCs.
RESULTS
There was a tendency to higher expression of HSP27 in the RCC than in normal renal tubular cells. Of the 48 RCCs, the HSP27 expression was positive in 38 cases. An inverse relationship was found between the Fuhrman nuclear grade and HSP27 expression, but this was without statistical significance (r=-0.218, p=0.093). No relationship between the HSP27 expression and the other parameters was observed. Also, no statistically significant difference was observed between apoptosis and the HSP27 expression more (p=0.951).
CONCLUSIONS
Although HSP27 expression was increased in RCC than in normal renal tubular cell the HSP27 expression may not be a powerful and statistically significant prognostic indicator in patients with RCC.
- Ipsilateral Synchronous Renal Cell Carcinoma and Transitional Cell Carcinoma: A Case Report.
- Ji Young Park, Eun Kyung Kwak, Tae In Park
- Korean J Pathol. 2002;36(6):429-432.
- 2,067 View
- 24 Download
-
 Abstract
Abstract
 PDF
PDF - We report a synchronous renal cell carcinoma (RCC) and renal pelvic transitional cell carcinoma (TCC) in the kidney of a 74-year-old man. The kidney was without hydronephrosis. The patient was admitted to the hospital because he had intermittent gross hematuria for three years. Histologically, a section of the specimen revealed a conventional (clear cell) RCC in renal parenchyma just beneath the renal pelvis and a papillary urothelial carcinoma arising from the renal pelvis at the upper pole; the two are completely separated from one another. The tumor cells of the TCC showed an overexpression of c-MET immunohistochemical staining and more intense positive reactivity for p53 immunohistochemical staining than those of the RCC. These findings suggest that c-Met and p53 may be associated with the development of papillary TCC.
- Cytologic Features of Renal Cell Carcinoma: Clear Cell, Granular Cell and Oncocytoma.
- Yeong Jin Choi, Youn Soo Lee, Mi Seon Kwon, Kyo Young Lee, Byung Kee Kim, Sang In Shim
- J Pathol Transl Med. 1996;7(1):31-37.
- 3,105 View
- 141 Download
-
 Abstract
Abstract
 PDF
PDF - It is well known that fine needle aspiration biopsy(FNAB) is very useful and has a high accuracy rate in the diagnosis of renal neoplasms. Although there is some indecision to perform the FNAB for a rare possibility of tumor seeding along the biopsy needle tract, it tends to be used increasingly. As in the cytologic diagnosis of metastatic lesion through out the body, renal cell carcinoma should nearly always be considered in the differential diagnosis, the precise understainding of cytologic features of renal cell carcinoma with various cell types and architectural patterns is necessarily required. In this report, we present three cases of primary renal cell tumors, two of renal cell carcinomas and one of oncocytoma, preponderantly emphasizing the cytologic differential points in the FNAB specimen.
- Epidermal Growth Factor Receptor Expression and Cell Proliferation in Renal Cell Carcinoma.
- Ji Shin Lee, Jong Jae Jung, Min Cheol Lee, Chang Soo Park
- Korean J Pathol. 2000;34(4):273-279.
- 2,121 View
- 19 Download
-
 Abstract
Abstract
 PDF
PDF - The epidermal growth factor receptor (EGFR) is a transmembrane glycoprotein whose expression is a possible cause of increased tumor cell proliferation and has recently been proposed as a prognostic parameter in some tumors. Expression of EGFR was studied immunohistochemically in 62 cases of human renal cell carcinomas to evaluate their possible prognostic roles. We also examined the correlation between EGFR expression and cell proliferation by immunohistochemical staining for proliferating cell nuclear antigen (PCNA). Fifty-six cases (90.3%) expressed EGFR, with staining largely confined to the cell membrane and cytoplasm. Staining intensity of EGFR was directly correlated with nuclear grade (p=0.000) and TNM stage (p=0.015). PCNA index was significantly higher in EGFR-positive tumors than in EGFR- negative tumors. There was a statistically significant positive correlation between PCNA index and increasing staining intensity of EGFR (p=0.000). In univariate survival analysis, EGFR expression was significantly associated with shortened survival. However, EGFR expression was not an independent prognostic factor by multivariate analysis. These findings suggest that EGFR expression may be an important cause of tumor cell proliferation in renal cell carcinoma and further studies are needed to evaluate whether EGFR expression analysis provides independent prognostic information.
- Sarcomatoid Renal Cell Carcinoma; Special Reference to its Distinction from Carcinosarcoma.
- Kee Taek Jang, Yeon Mee Kim, Je Geun Chi
- Korean J Pathol. 1998;32(5):378-381.
- 1,824 View
- 10 Download
-
 Abstract
Abstract
- Sarcomatoid renal cell carcinoma is an uncommon tumor that has to be distinguished from renal carcinosarcoma. We have described three cases of sarcomatoid renal cell carcinoma showing different clinical and light microscopic features. An ultrastructural study of the tumor cells from the sarcomatoid area revealed frequent desmosomal junction, confirming the epithelial nature of the neoplasm. All three cases showed an aggressive clinical course and tended to invade adjacent organs or tissues. We believe that an histological and immunohistochemical examination in conjunction with an electron microscopic examination are necessary to diagnose sarcomatoid renal cell carcinoma.
- Osteopontin Expression and Its Prognostic Significances in Human Renal Cell Carcinoma.
- Hee Yeon Hong, Hyang Lan Lee, Tae Sook Kim, Ghil Suk Yoon
- Korean J Pathol. 2006;40(3):225-230.
- 2,152 View
- 25 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Osteopontin (OPN) is a glycoprotein and it participates in cell-cell and cell-matrix interactions. In vitro studies suggest that the OPN expression is associated with tumor metastasis, and especially with the metastasis of osteotropic tumors originating in breast, prostate and lungs. Since no human tissue study has suggested the means by which OPN participates in the tumorigenesis, angiogenesis, progression and metastasis of renal cell carcinoma (RCC), we evaluated the expression and prognostic significance of OPN in RCC.
METHODS
Immunohistochemistry was performed with using the primary antibody for OPN on the archival paraffin-embedded tissue microarray specimens from 51 RCC patients who underwent radical or simple nephrectomy.
RESULTS
In the normal kidney specimens, OPN was expressed in a few compressed distal tubules adjacent to the RCCs. In RCCs, the OPN expression was elevated in larger tumors (p<0.05) and in the tumor with low microvessel density (p<0.01). In the present study, univariate analysis indicated that stage, tumor size, lymph node and distant organ metastasis are significant prognostic factors for disease free survival (DFS) in RCC patients (p<0.01), but OPN is not (p=0.0661). Multivariate analysis indicated lymph node metastasis is the independent prognostic indicator of DFS (p<0.05).
CONCLUSION
Though this study has statistical limitations, these results suggest OPN plays a role in tumor progression and metastasis and it may act as a potential prognostic indicator to predict the prognosis of RCC patients.
- Histopathology and Mainz Classification of Renal Cell Tumors: A Histogenetic Study and DNA Content Analysis.
- Yeong Jin Choi, Tae Kon Hwang, Youn Soo Lee, Byung Kee Kim, Sun Moo Kim, Sang In Shim
- Korean J Pathol. 1998;32(7):511-520.
- 2,289 View
- 10 Download
-
 Abstract
Abstract
- The Mainz classification for renal cell tumors was introduced in 1986 and it's utility has been reported in several histogenetic and genetic studies of renal cell tumors. We present a study of 127 cases of renal cell tumors with clinicopathologic correlation, DNA content analysis, and histogenesis studied by histochemical and immunohistochemical staining. The 127 renal cell tumors classified by the Mainz classification were 87 clear cell, 17 chromophilic, 13 chromophobe and 3 sarcomatoid renal cell carcinomas, 5 oncocytomas and 2 adenomas. These subtypes showed significant correlation not with age, sex, Robson's stage, DNA ploidy or tumor recurrence but with nuclear grade (p=0.001) and tumor size (p=0.001). Hall's colloidal iron (p=0.002) and carbonic anhydrase II (p=0.013) stains, representing the origin of distal nephron especially of collecting duct, were significantly correlated with specific subtypes of renal cell tumors, especially chromophobe cell renal carcinoma. This study demonstrates that the Mainz classification suggests several morphologically different subtypes and variants of renal cell tumors and that some of them may have originated from the distal nephron, particularly from the collecting duct.
- Analysis of Gene Expression in Renal Cell Carcinomas Using cDNA Microarray: Reduced Expression of Decorin in Renal Cell Carcinomas.
- Jin Sook Lee, Kang Suek Suh, Kyung Un Choi, Jee Yeun Kim, Do Youn Park
- Korean J Pathol. 2003;37(4):232-238.
- 1,886 View
- 14 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Identification of the genes expressed differentially in renal cell carcinoma (RCC)but not in the non-cancerous kidney is important for understanding the molecular basis ofrenal cell carcinoma and for defining possible prognostic value and therapeutic intervention.We investigated the changes in gene expression accompanying the development and progression of kidney cancer by cDNA microarrays.
METHODS
To identify molecular alterations in renal cell carcinoma, we measured expression profiles for paired neoplastic and noncancerouskidney samples from an individual by means of a cDNA microarry representing 7, 500genes. Of the differentially expressed genes, we assessed the decorin gene at the proteinlevel using immunohistochemistry.
RESULTS
The 60 genes were noted to have more than a fivefold change in expression (either increased or decreased) in RCC compared to the noncancerouskidney. The changed genes are those associated with signal transduction, metabolizingenzymes, the cytoskeleton, cell adhesion, cell cycle control, modulation of transcription, the tumor suppressor gene and tumor antigens. Under immunohistochemistry, the expressionof decorin was significantly decreased in the tumor than in the non-cancerous kidney.The expression rate of decorin was not associated with the patient's sex, age, histologic type, Fuhrmann nuclear grade and T stage.
CONCLUSION
The author predicted that these geneexpression profiling experiments will lead to improvements in the basic understanding of renaltumor pathogenesis and will promote the discovery of novel molecular markers for renal tumordiagnosis and therapy.
- Multilocular Cystic Renal Cell Carcinoma: A case report.
- Ki Jung Yun, Weon Cheol Han, Chan Choi, Hyung Bae Moon, Joung Sik Rim
- Korean J Pathol. 1992;26(3):314-316.
- 2,024 View
- 17 Download
-
 Abstract
Abstract
 PDF
PDF - Multilocular cystic renal cell carcinoma appears to be a distinct and rare subtype of renal cell carcinoma with characteristic gross and microscopic features. Multilocular cystic renal cell carcinoma should be separated from multilocular cyst, multilocular cystic nephroma, and renal cell carcinoma with cystic degeneration. We present a case of multilocular cystic renal cell carcinoma. A 61-year-old man presented with right flank pain for 4 years. The computerized tomography revealed multilocular cystic mass in the upper pole of right kidney. The cystic mass measured 4.5x4 cm. The cyst was multilocular and locules not communicated with each other. The solid area was not present. Microscopically, the locules were lined by flat or cuboidal neoplastic clear cells. The clear cells were focally aggregated in the septa. The nephron was not present in the septa.
- Eosinophilic Cytoplasmic Globules in Papillary Renal Cell Carcinoma: A Case Report.
- Ok Ran Shin, Jae Young Park, Hae Kyung Lee, Young Seok Lee, Chang Hee Han, Sung Hak Kang, Kyo Young Lee, Yeong Jin Choi
- Korean J Pathol. 2006;40(6):466-468.
- 2,387 View
- 27 Download
-
 Abstract
Abstract
 PDF
PDF - Eosinophilic cytoplasmic globules may be seen in a variety of neoplastic and nonneoplastic conditions and are most often associated with alpha-1-antitrypsin deficiency, several pathologic liver conditions and yolk sac tumors. A few cases of eosinophilic cytoplasmic globules in renal cell carcinoma have been reported but there has only been one report of papillary type. We report another case of papillary renal cell carcinoma with eosinophilic cytoplasmic globules, which is similar to a Mallory body but with different properties.

 E-submission
E-submission


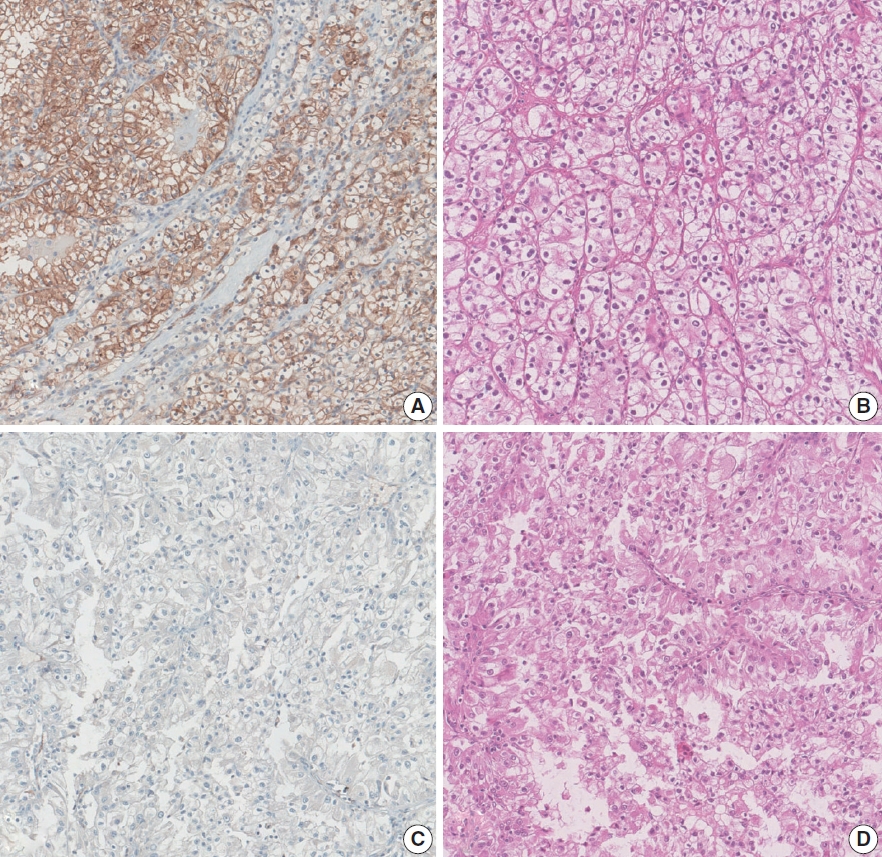
 First
First Prev
Prev



