Search
- Page Path
- HOME > Search
- Characterization of undifferentiated carcinoma of the salivary gland: clinicopathological and immunohistochemical analyses in comparison with lymphoepithelial carcinoma
- Sangjoon Choi, Gyuheon Choi, Hee Jin Lee, Joon Seon Song, Yoon Se Lee, Seung-Ho Choi, Kyung-Ja Cho
- J Pathol Transl Med. 2025;59(6):361-370. Published online September 8, 2025
- DOI: https://doi.org/10.4132/jptm.2025.07.07
- 2,790 View
- 258 Download
-
 Abstract
Abstract
 PDF
PDF - Background
This study aimed to reclassify a subset of poorly differentiated salivary gland carcinoma that do not conform to any entities of the current World Health Organization (WHO) classification into the category of undifferentiated carcinoma (UDC) because they lack specific histologic differentiation or immunophenotype. Methods: Cases of salivary gland carcinomas from Asan Medical Center (2002–2020) that did not fit any existing WHO classification criteria and were diagnosed as poorly differentiated carcinoma, high-grade carcinoma, or UDC, were retrospectively reviewed. Immunohistochemical (IHC) staining for p40, neuroendocrine markers, androgen receptor (AR), and gross cystic disease fluid protein 15 (GCDFP-15) and Epstein-Barr virus (EBV) in situ hybridization (ISH) were performed. Clinical data were collected from the electronic medical records. Results: Six salivary gland carcinomas did not align with any specific entities and lacked distinct differentiation. Two of six cases displayed lymphoepithelial carcinoma (LEC)-like morphology but were negative or showed negligible immunoreactivity for p40 and EBV ISH, distinguishing them from LEC of the salivary gland. Two cases showed strong AR positivity, suggesting a potential overlap with salivary duct carcinoma (SDC) but lacked classic SDC morphologies and GCDFP-15 expression. No cases expressed neuroendocrine markers. Conclusions: This study proposes reclassifying these poorly differentiated or high-grade salivary gland carcinomas as UDC based on their indeterminate differentiation and IHC profiles. This may lead to a clearer diagnostic category and enhance our understanding of these high-grade tumors.
- Primary Merkel cell carcinoma of the salivary gland: a clinicopathologic study of four cases with a review of literature
- Gyuheon Choi, Joon Seon Song, Hee Jin Lee, Gi Hwan Kim, Young Ho Jung, Yoon Se Lee, Kyung-Ja Cho
- J Pathol Transl Med. 2025;59(3):171-179. Published online April 30, 2025
- DOI: https://doi.org/10.4132/jptm.2025.03.25
- 3,580 View
- 155 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Primary Merkel cell carcinoma of the salivary gland is currently not listed in the World Health Organization classification. However, cases of Merkel cell type neuroendocrine carcinomas of the salivary gland with perinuclear cytokeratin 20 positivity have been intermittently reported. We here investigated the clinicopathologic features of additional cases.
Methods
Data of four cases of Merkel cell type small cell neuroendocrine carcinoma of the salivary gland were retrieved. To confirm the tumors’ primary nature, clinical records and pathologic materials were reviewed. Optimal immunohistochemical staining was performed to support the diagnosis.
Results
All tumors were located in the parotid gland. Possibilities of metastasis were excluded in all cases through a meticulous clinicopathological review. Tumor histology was consistent with the diagnosis of small cell neuroendocrine carcinoma. Tumors’ immunohistochemical phenotypes were consistent with Merkel cell carcinoma, including Merkel cell polyomavirus large T antigen positivity in two of the four cases.
Conclusions
Merkel cell carcinomas can originate in salivary glands and are partly associated with Merkel cell polyomavirus infection as in cutaneous Merkel cell carcinomas. -
Citations
Citations to this article as recorded by- Parotid intranodal metastasis of Merkel cell carcinoma: a rare case report
Tong Gao, Dengshun Wang, Hongwei Yu, Yu’e Wang, Haibin Lu
BMC Oral Health.2025;[Epub] CrossRef
- Parotid intranodal metastasis of Merkel cell carcinoma: a rare case report
- Adenocarcinoma of the minor salivary gland with concurrent MAML2 and EWSR1 alterations
- Sangjoon Choi, Junhun Cho, Seung Eun Lee, Chung-Hwan Baek, Yi-Kyung Kim, Hyung-Jin Kim, Young Hyeh Ko
- J Pathol Transl Med. 2021;55(2):132-138. Published online January 22, 2021
- DOI: https://doi.org/10.4132/jptm.2020.12.11
- 7,365 View
- 126 Download
- 12 Web of Science
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF - Salivary gland tumors are histologically diverse, and each entity has distinctive histopathological and molecular features. We report two cases of salivary gland tumors with unique histological and molecular findings, which have not been documented previously. The tumors were located in the base of the tongue in both patients. Most tumor cells were arranged in cords and nests, giving a trabecularlike appearance. Focally, glandular structures with intraluminal mucin and perivascular pseudorosette-like configurations were identified. Tumor cells had eosinophilic to clear cytoplasm, and showed mild nuclear atypia. They were positive for pancytokeratin and negative for S-100, p63, c-KIT, androgen receptor, and neuroendocrine markers. Multiple foci of capsular or lymphovascular invasion were identified, but the Ki-67 labeling index was low (< 5%). Fluorescence in situ hybridization revealed concurrent alterations of MAML2 and EWSR1 gene. Further investigations with a larger number of cases with similar histological and molecular features will accurately classify this tumor.
-
Citations
Citations to this article as recorded by- A novel fusion of EWSR1::PRKD1 in cribriform adenocarcinoma of salivary glands: A rare case report
Zecra Yahia
Human Pathology Reports.2025; 42: 300801. CrossRef - Salivary Gland Neoplasms With a Unique Trabecular Histology and MAML2 Translocation
Bokyung Ahn, Seung-Ho Choi, Doeun Kim, Deokhoon Kim, Kyung-Ja Cho
American Journal of Surgical Pathology.2023; 47(10): 1085. CrossRef - Mesonephric-like Adenocarcinoma of the Ovary: Clinicopathological and Molecular Characteristics
Hyun Hee Koh, Eunhyang Park, Hyun-Soo Kim
Diagnostics.2022; 12(2): 326. CrossRef - The evolving role of molecular pathology in the diagnosis of salivary gland tumours with potential pitfalls
Kanwalpreet Kaur, Shailee Mehta, Sangita Vanik, Priti Trivedi, Nirmalya Banerjee, Harsh Dhar, Sourav Datta, Subhadeep Karanjai
European Archives of Oto-Rhino-Laryngology.2022; 279(8): 3769. CrossRef - Alveolar Soft Part Sarcoma of the Uterus: Clinicopathological and Molecular Characteristics
Yurimi Lee, Kiyong Na, Ha Young Woo, Hyun-Soo Kim
Diagnostics.2022; 12(5): 1102. CrossRef - Endometrioid Carcinomas of the Ovaries and Endometrium Involving Endocervical Polyps: Comprehensive Clinicopathological Analyses
Jihee Sohn, Yurimi Lee, Hyun-Soo Kim
Diagnostics.2022; 12(10): 2339. CrossRef - Mesonephric-like Differentiation of Endometrial Endometrioid Carcinoma: Clinicopathological and Molecular Characteristics Distinct from Those of Uterine Mesonephric-like Adenocarcinoma
Sujin Park, Go Eun Bae, Jiyoung Kim, Hyun-Soo Kim
Diagnostics.2021; 11(8): 1450. CrossRef - Mesonephric-like Adenocarcinoma of the Uterine Corpus: Comprehensive Immunohistochemical Analyses Using Markers for Mesonephric, Endometrioid and Serous Tumors
Hyunjin Kim, Kiyong Na, Go Eun Bae, Hyun-Soo Kim
Diagnostics.2021; 11(11): 2042. CrossRef
- A novel fusion of EWSR1::PRKD1 in cribriform adenocarcinoma of salivary glands: A rare case report
- Primary squamous cell carcinoma of the salivary gland: immunohistochemical analysis and comparison with metastatic squamous cell carcinoma
- Uiree Jo, Joon Seon Song, Seung-Ho Choi, Soon Yuhl Nam, Sang Yoon Kim, Kyung-Ja Cho
- J Pathol Transl Med. 2020;54(6):489-496. Published online August 31, 2020
- DOI: https://doi.org/10.4132/jptm.2020.07.19
- 10,145 View
- 207 Download
- 18 Web of Science
- 18 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
Primary squamous cell carcinoma (SCC) of the salivary gland is a rare disease, and distinguishing primary SCC from metastatic SCC is difficult. This study investigated the histological and immunohistochemical differences between primary and metastatic salivary gland SCC to improve the accuracy of diagnosis and to explore the pathogenesis of this disease.
Methods
Data of 16 patients who underwent surgery for SCC of salivary glands between 2000 and 2018 at Asan Medical Center were retrieved. Eight patients had a history of SCC at other sites, and eight patients had only salivary gland SCC. Immunostaining for p16, p53, androgen receptor (AR), gross cystic disease fluid protein 15 (GCDFP-15), and c-erbB2, as well as mucicarmine staining, were compared between the two groups.
Results
Most tumors were located in the center of the salivary glands with extraparenchymal extension. The histology of primary SCC of the salivary gland was consistent with moderately differentiated SCC with extensive desmoplastic reaction and peritumoral inflammation. Involvement of the salivary gland ducts and transition into the ductal epithelium were observed in two cases. Metastatic SCC resembled the primary tumor histologically and was associated with central necrosis. Both groups exhibited negative mucin staining. Two, one, and one primary SCC case exhibited AR, GCDFP-15, and c-erbB2 positivity, respectively.
Conclusions
A subset of primary SCCs originated in salivary ducts or was related to salivary duct carcinoma. Distinguishing primary from metastatic SCC of the salivary gland is difficult using histologic features and immunoprofiles. A comprehensive review of the medical history is essential. -
Citations
Citations to this article as recorded by- Clinical diagnosis, treatment, and survival analysis of 61 cases of salivary duct carcinoma: a retrospective study
Shubin Dong, Mengru Li, Zhiwei Zhang, Bowei Feng, Wei Ding, Jiang Chang, Feng Liu
PeerJ.2025; 13: e19626. CrossRef - Characterization of undifferentiated carcinoma of the salivary gland: clinicopathological and immunohistochemical analyses in comparison with lymphoepithelial carcinoma
Sangjoon Choi, Gyuheon Choi, Hee Jin Lee, Joon Seon Song, Yoon Se Lee, Seung-Ho Choi, Kyung-Ja Cho
Journal of Pathology and Translational Medicine.2025; 59(6): 361. CrossRef - Primary salivary gland squamous cell carcinoma with sialolithiasis in the submandibular gland: A case report and literature review
Sawako Ono, Katsutoshi Hirose, Yuji Hirata, Marie Yamada, Satoko Nakamura, Hidetaka Yamamoto
Journal of Oral and Maxillofacial Surgery, Medicine, and Pathology.2024; 36(5): 768. CrossRef - A case of primary squamous cell carcinoma of the parotid gland and review of the literature
Jingli Zhao, Xinrong Nan, Chuhuan Zhou, Nan Jiang, Liangliang Tian
Journal of Case Reports and Images in Oncology.2024; 10(1): 7. CrossRef - Metastatic cutaneous squamous cell carcinoma accounts for nearly all squamous cell carcinomas of the parotid gland
Patrick J. Bradley, Göran Stenman, Lester D. R. Thompson, Alena Skálová, Roderick H. W. Simpson, Pieter J. Slootweg, Alessandro Franchi, Nina Zidar, Alfons Nadal, Henrik Hellquist, Michelle D. Williams, Ilmo Leivo, Abbas Agaimy, Alfio Ferlito
Virchows Archiv.2024; 485(1): 3. CrossRef - Common skin cancers and their association with other non-cutaneous primary malignancies: a review of the literature
Lindsay Holic
Medical Oncology.2024;[Epub] CrossRef - Salivary duct carcinoma with squamous differentiation: histomorphological and immunophenotypical analysis of six cases
Melad N Dababneh, Christopher C Griffith, Kelly R Magliocca, Ivan J Stojanov
Histopathology.2024; 85(4): 590. CrossRef - Comprehensive Next Generation Sequencing Reveals that Purported Primary Squamous Cell Carcinomas of the Parotid Gland are Genetically Heterogeneous
Justin A. Bishop, Masato Nakaguro, Ilan Weinreb, Doreen Palsgrove, Lisa M. Rooper, Travis W. Vandergriff, Brian Carlile, Jeffrey A. Sorelle, Jeffrey Gagan, Toshitaka Nagao
Head and Neck Pathology.2024;[Epub] CrossRef - Salivary gland fine needle aspiration: a focus on diagnostic challenges and tips for achieving an accurate diagnosis
Carla Saoud, Hansen Lam, Sandra I. Sanchez, Zahra Maleki
Diagnostic Histopathology.2023; 29(8): 357. CrossRef - Salivary gland pathologies: evolution in classification and association with unique genetic alterations
Michał Żurek, Łukasz Fus, Kazimierz Niemczyk, Anna Rzepakowska
European Archives of Oto-Rhino-Laryngology.2023; 280(11): 4739. CrossRef - A retrospective study of nonneoplastic and neoplastic disorders of the salivary glands
Sorin Vamesu, Oana Andreea Ursica, Ana Maria Gurita, Raluca Ioana Voda, Mariana Deacu, Mariana Aschie, Madalina Bosoteanu, Georgeta Camelia Cozaru, Anca Florentina Mitroi, Cristian Ionut Orasanu
Medicine.2023; 102(42): e35751. CrossRef - Pembrolizumab as a first line therapy in a patient with extensive mucoepidermoid salivary gland carcinoma. A complete clinical, radiological and pathological response. A very specific case
Raed Farhat, Noam Asna, Yaniv Avraham, Ashraf Khater, Majd Asakla, Alaa Safia, Sergio Szvalb, Nidal Elkhatib, Shlomo Merchavy
Discover Oncology.2022;[Epub] CrossRef - Morphologic CT and MRI features of primary parotid squamous cell carcinoma and its predictive factors for differential diagnosis with mucoepidermoid carcinoma
Xiaohua Ban, Huijun Hu, Yue Li, Lingjie Yang, Yu Wang, Rong Zhang, Chuanmiao Xie, Cuiping Zhou, Xiaohui Duan
Insights into Imaging.2022;[Epub] CrossRef - A Rare Case of Primary Squamous Cell Carcinoma of the Submandibular Salivary Gland: Brief Overview of Diagnostic Ambiguity and Treatment Challenges
Pawan Hingnikar, Anendd Jadhav, Nitin D Bhola
Cureus.2022;[Epub] CrossRef - Necrotizing Sialometaplasia of the Hard Palate: Diagnosis and
Treatment
Sangeun Lee, Yun Sung Lim, Kyuho Lee, Bo Hae Kim
Journal of Clinical Otolaryngology Head and Neck Surgery.2022; 33(4): 236. CrossRef - Parotid Salivary Duct Carcinoma With a Prominent Squamous Component: Immunohistochemical Profile, Diagnostic Pitfalls, and Therapeutic Implications
Naomi Hardy, Joshua Thompson, Ranee Mehra, Cinthia B. Drachenberg, Kyle Hatten, John C. Papadimitriou
International Journal of Surgical Pathology.2021; 29(7): 726. CrossRef - Intrasalivary Thymic Carcinoma: A Case Report and Literature Review
Michał Kunc, Alexandra Kamieniecki, Grzegorz Walczak, Tomasz Nowicki, Bartosz Wasąg, Bogusław Mikaszewski, Dominik Stodulski, Wojciech Biernat
Head and Neck Pathology.2021; 16(3): 857. CrossRef - Cancer Stem Cell Markers in Squamous Cell Carcinomas of the Salivary Glands
Mattis Bertlich, Julia Kitz, Marie Kruizenga, Jennifer Lee Spiegel, Martin Canis, Friedrich Ihler, Frank Haubner, Bernhard G. Weiss, Mark Jakob
Oncology.2021; 99(6): 402. CrossRef
- Clinical diagnosis, treatment, and survival analysis of 61 cases of salivary duct carcinoma: a retrospective study
- A retrospective cytohistological correlation of fine-needle aspiration cytology with classification by the Milan System for Reporting Salivary Gland Cytopathology
- Ji Hyun Park, Yoon Jin Cha, Ja Yeong Seo, Jae Yol Lim, Soon Won Hong
- J Pathol Transl Med. 2020;54(5):419-425. Published online July 8, 2020
- DOI: https://doi.org/10.4132/jptm.2020.06.09
- 7,036 View
- 201 Download
- 11 Web of Science
- 14 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Before publication of the new classification system named the Milan System for Reporting Salivary Gland Cytopathology (MSRSGC) in 2018, there was no standard classification for salivary gland lesions obtained by fine-needle aspiration (FNA). We therefore aimed to evaluate the diagnostic utility of this system by retrospectively reviewing FNA samples using the MSRSGC and to determine their risk of developing into neoplasms and becoming malignant.
Methods
Retrospective slide review and classification of salivary gland FNAs obtained over a 6-year period (2013–2018) at a single center were performed by two pathologists. The risks of neoplasm and malignancy for each category also were calculated.
Results
This study surveyed 374 FNAs (371 patients) performed over a six-year period and selected 148 cases that included documented surgical follow-up (39.6%). Among the surgically treated cases, the distributions of FNA categories were as follows: non-diagnostic (ND; 16.9%), non-neoplastic (NN; 2.7%), atypia of undetermined significance (AUS; 3.4%), benign (BN; 54.7%), salivary gland neoplasm of uncertain malignant potential (SUMP; 10.1%), suspicious for malignancy (SM; 6.8%), and malignant (M; 5.4%). The risk of malignancy (ROM) was 24.0% for ND, 0% for NN, 40.0% for AUS, 2.5% for BN, 46.7% for SUMP, 100% for SM, and 87.5% for M. The overall diagnostic accuracy was 95.9% (142/148 cases).
Conclusions
The newly proposed MSRSGC appears to be a reliable system for classification of salivary gland lesions according to the associated ROM. -
Citations
Citations to this article as recorded by- The Impact of Lesion-Specific and Sampling-Related Factors on Success of Salivary Gland Fine-Needle Aspiration Cytology
Marcel Mayer, Mohammad Marwan Alfarra, Kathrin Möllenhoff, Marianne Engels, Christoph Arolt, Alexander Quaas, Philipp Wolber, Louis Jansen, Lisa Nachtsheim, Maria Grosheva, Jens Peter Klussmann, Sami Shabli
Head and Neck Pathology.2025;[Epub] CrossRef - The Myriad Spectrum of Salivary Gland Lesions: Cytohistological Correlation on Fine Needle Aspiration Cytology, Core Needle Biopsy, and Resections in a 5‐Year Single Institutional Experience of North India
Zachariah Chowdhury, Pallavi Majumdar, Sumeet Narain, Komal Lamba
Diagnostic Cytopathology.2025; 53(8): 391. CrossRef - Diagnostic Performance of the Milan System for Reporting Salivary Gland Cytopathology and a Proposed Algorithm for Fine-Needle Aspiration Cytology of Salivary Gland Lesions
Norihide Mochizuki, Hirotaka Fujita, Takuma Tajiri, Masataka Ueda, Makiko Kurata, Chie Inomoto, Tomoko Sugiyama, Daisuke Maki, Shuichi Shiraishi, Tomohisa Machida, Hitoshi Ito, Yohei Masugi, Naoya Nakamura
Acta Cytologica.2025; 69(4): 324. CrossRef - A study of fine needle aspiration cytology and histopathology correlation of salivary gland neoplasms in a tertiary care hospital: an observational study
Asima Malik, Ahlam Mushtaq, Salma Bhat, Suhail Naik
International Journal of Contemporary Pediatrics.2025; 13(1): 23. CrossRef - The Milan system for reporting salivary gland cytopathology – Assessment of utility and the risk of malignancy
Annu E. Prakash, Renu Sukumaran, Nileena Nayak, K. Lakshmi, Anitha Mathews, Jayasree Kattoor
Indian Journal of Cancer.2024; 61(3): 575. CrossRef - Salivary gland fine-needle aspiration biopsy: quality assurance results from a tertiary cancer center
Fanni Ratzon, Dominique L. Feliciano, Nora Katabi, Bin Xu, Oscar Lin, Xiao-Jun Wei
Journal of the American Society of Cytopathology.2023; 12(3): 206. CrossRef - Cytohistological correlation and risk stratification of salivary gland lesions using the Milan System for Reporting Salivary Gland Cytopathology: A tertiary care centre experience
Tarun Kumar, Prerna Tewari, Jitendra Singh Nigam, Shreekant Bharti, Surabhi, Ruchi Sinha, Punam Prasad Bhadani
Cytopathology.2023; 34(3): 225. CrossRef - Assessment of Risk of Malignancy of Fine-needle Aspiration Cytology in Salivary Gland Lesions Using the Milan System for Reporting Salivary Gland Cytopathology Categorization: A Systematic Review and Meta-analysis
Amit Kumar, Subhash Chandra, Bishnupati Singh, Swati Sharma, Ankita Tandon, Ajoy Kumar Shahi
The Journal of Contemporary Dental Practice.2023; 23(10): 1039. CrossRef - Milan Sınıflandırma Sistemi’ne Göre Değerlendirilen Tükürük Bezi İnce İğne Aspirasyon Sitolojilerinin Histopatolojik Tanı Uyumu
Özlem SARAYDAROĞLU, Selin YİRMİBEŞ
Uludağ Üniversitesi Tıp Fakültesi Dergisi.2023; 49(3): 285. CrossRef - Milan system for reporting salivary gland cytopathology: Adoption and outcomes in a community setting
Samih J. Nassif, Ali R. Sasani, Garrey T. Faller, Jennifer L. Harb, Jagdish K. Dhingra
Head & Neck.2022; 44(6): 1462. CrossRef - Nondiagnostic salivary gland FNAs are associated with decreased risk of malignancy compared with “all‐comer” patients: Analysis of the Milan System for Reporting Salivary Gland Cytopathology with a focus on Milan I: Nondiagnostic
Shu K. Lui, Troy Tenney, Patrick C. Mullane, Kartik Viswanathan, Daniel J. Lubin
Cancer Cytopathology.2022; 130(10): 800. CrossRef - Application of the Milan System for Reporting Salivary Gland Cytopathology: A systematic review and meta‐analysis
Zhaoyang Wang, Huan Zhao, Huiqin Guo, Changming An
Cancer Cytopathology.2022; 130(11): 849. CrossRef - Multiplexed single‐cell analysis of FNA allows accurate diagnosis of salivary gland tumors
Juhyun Oh, Tae Yeon Yoo, Talia M. Saal, Lisa Tsay, William C. Faquin, Jonathan C.T. Carlson, Daniel G. Deschler, Sara I. Pai, Ralph Weissleder
Cancer Cytopathology.2022; 130(8): 581. CrossRef - Cytologic analysis of vitreous fluids: A retrospective review of our 24 years of experience
Gabriel L. Collins, Elizabeth W. Hubbard, Christopher T. Clark, Lisa D. Duncan, Laurentia Nodit
Diagnostic Cytopathology.2021; 49(10): 1122. CrossRef
- The Impact of Lesion-Specific and Sampling-Related Factors on Success of Salivary Gland Fine-Needle Aspiration Cytology
- Primary Necrobiotic Xanthogranulomatous Sialadenitis with Submandibular Gland Localization without Skin Involvement
- Myunghee Kang, Na Rae Kim, Dong Hae Chung, Jae Yeon Seok, Dong Young Kim
- J Pathol Transl Med. 2019;53(4):261-265. Published online January 16, 2019
- DOI: https://doi.org/10.4132/jptm.2019.01.08
- 8,951 View
- 169 Download
- 2 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF - Necrobiotic xanthogranulomatous reaction is a multiorgan, non-Langerhans cell histiocytosis with an unknown etiology. Occurrence in the salivary gland is extremely rare. We recently identified a case of necrobiotic xanthogranulomatous sialadenitis in a 73-year-old Korean woman who presented with a painless palpable lesion in the chin. There was no accompanying cutaneous lesion. Partial resection and subsequent wide excision with neck dissection were performed. Pathological examination showed a severe inflammatory lesion that included foamy macrophages centrally admixed with neutrophils, eosinophils, lymphocytes, plasma cells, and scattered giant cells, as well as necrobiosis. During the 12-month postoperative period, no grossly remarkable change in size was noted. Necrobiotic xanthogranulomatous inflammation may be preceded by or combined with hematologic malignancy. Although rare, clinicians and radiologists should be aware that an adhesive necrobiotic xanthogranuloma in the salivary gland may present with a mass-like lesion. Further evaluation for hematologic disease and close follow-up are needed when a pathologic diagnosis is made.
-
Citations
Citations to this article as recorded by- Salivary gland macrophages in health and disease: heterogeneity, niche crosstalk, and therapeutic avenues
Xinglei Li, Yan Feng, Huixin Xue, Xinxin Ni
Frontiers in Immunology.2025;[Epub] CrossRef - Five Cases of Xanthogranulomatous Sialadenitis
Satoshi Kiyama, Hiroyuki Iuchi, Kotoko Ito, Kengo Nishimoto, Tsutomu Matsuzaki, Masaru Yamashita
Practica Oto-Rhino-Laryngologica.2022; 115(4): 315. CrossRef - Xanthogranulomatous change in a pleomorphic adenoma: An extremely rare variant/degenerative change. Is it fine needle aspiration induced?
Mukta Pujani, Dipti Sidam, Kanika Singh, Aparna Khandelwal, Khushbu Katarya
Diagnostic Cytopathology.2021;[Epub] CrossRef - A Case of Xanthogranulomatous Sialadenitis with Facial Palsy Mimicking Malignancy
Sang Hyun Kim, Sun Woo Kim, Sang Hyuk Lee
Korean Journal of Otorhinolaryngology-Head and Neck Surgery.2021; 64(6): 422. CrossRef - Xanthogranulomatous Sialadenitis, an Uncommon Reactive Change is Often Associated with Warthin’s Tumor
Lihong Bu, Hui Zhu, Emilian Racila, Sobia Khaja, David Hamlar, Faqian Li
Head and Neck Pathology.2020; 14(2): 525. CrossRef - A Case of Xanthogranulomatous Sialadenitis of the Sublingual Gland:A Review of Literature
Naoya KITAMURA, Seiji OHNO, Tetsuya YAMAMOTO
Journal of Japanese Society of Oral Medicine.2019; 25(1): 20. CrossRef
- Salivary gland macrophages in health and disease: heterogeneity, niche crosstalk, and therapeutic avenues
- Cytopathologic Features of Secretory Carcinoma of Salivary Gland: Report of Two Cases
- Young Ah Kim, Jae Won Joung, Sun-Jae Lee, Hoon-Kyu Oh, Chang Ho Cho, Woo Jung Sung
- J Pathol Transl Med. 2019;53(1):70-74. Published online December 28, 2018
- DOI: https://doi.org/10.4132/jptm.2018.11.09
- 8,261 View
- 147 Download
- 7 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF - Secretory carcinoma of the salivary gland (SC) is a newly introduced rare salivary gland tumor that shares histological, immunohistochemical, and genetic characteristics with secretory carcinoma of the breast. Here, we report the cytologic features of two cases of SC confirmed by surgical resection. In these two cases, SC was incidentally detected in a 64-year-old female and a 56-yearold male. Fine needle aspiration cytology revealed nests of tumor cells with a papillary or glandular structure floating in mucinous secretions. The tumor cells demonstrated uniform, round, smooth nuclear contours and distinct nucleoli. Multiple characteristic cytoplasmic vacuoles were revealed. Singly scattered tumor cells frequently showed variable sized cytoplasmic vacuoles. The cytopathologic diagnosis of SC should be considered when characteristic cytological findings are revealed. Further immunohistochemistry and gene analyses are helpful to diagnose SC.
-
Citations
Citations to this article as recorded by- Salivary Gland Secretory Carcinoma; Review of 13 Years World‐Wide Experience and Meta‐Analysis
Eyal Yosefof, Tomer Boldes, Daniel Dan, Eyal Robenshtok, Yulia Strenov, Gideon Bachar, Thomas Shpitzer, Aviram Mizrachi
The Laryngoscope.2024; 134(4): 1716. CrossRef - Efficacy of Fine-Needle Aspiration Cytology in Diagnosing Secretory Carcinoma of Salivary Gland: A Systematic Review and Meta-Analysis
Pooja Sharma Kala, Mamta Gupta, Naveen Thapliyal
Acta Cytologica.2024; 68(2): 83. CrossRef - An Underappreciated Cytomorphological Feature of Secretory Carcinoma of Salivary Gland on Fine Needle Aspiration Biopsy: Case Report with Literature Review
Yinan Hua, Bing Leng, Kenneth E. Youens, Lina Liu
Head and Neck Pathology.2022; 16(2): 567. CrossRef - Prognostic factors in mammary analogue secretory carcinomas of the parotid gland: Systematic review and meta‐analysis
Stefan Janik, Muhammad Faisal, Blazen Marijić, Stefan Grasl, Matthaeus Ch. Grasl, Gregor Heiduschka, Boban M. Erovic
Head & Neck.2022; 44(3): 792. CrossRef - A systematic review of secretory carcinoma of the salivary gland: where are we?
Lísia Daltro Borges Alves, Andreia Cristina de Melo, Thayana Alves Farinha, Luiz Henrique de Lima Araujo, Leandro de Souza Thiago, Fernando Luiz Dias, Héliton Spíndola Antunes, Ana Lucia Amaral Eisenberg, Luiz Claudio Santos Thuler, Daniel Cohen Goldember
Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology.2021; 132(4): e143. CrossRef - Clinical characteristics of acinic cell carcinoma and secretory carcinoma of the parotid gland
Tetsuya Terada, Ryo Kawata, Keiki Noro, Masaaki Higashino, Shuji Nishikawa, Shin-ichi Haginomori, Yoshitaka Kurisu, Hiroko Kuwabara, Yoshinobu Hirose
European Archives of Oto-Rhino-Laryngology.2019; 276(12): 3461. CrossRef
- Salivary Gland Secretory Carcinoma; Review of 13 Years World‐Wide Experience and Meta‐Analysis
- PLAG1, SOX10, and Myb Expression in Benign and Malignant Salivary Gland Neoplasms
- Ji Hyun Lee, Hye Ju Kang, Chong Woo Yoo, Weon Seo Park, Jun Sun Ryu, Yuh-Seog Jung, Sung Weon Choi, Joo Yong Park, Nayoung Han
- J Pathol Transl Med. 2019;53(1):23-30. Published online November 14, 2018
- DOI: https://doi.org/10.4132/jptm.2018.10.12
- 12,646 View
- 382 Download
- 27 Web of Science
- 33 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Recent findings in molecular pathology suggest that genetic translocation and/oroverexpression of oncoproteins is important in salivary gland tumorigenesis and diagnosis. Weinvestigated PLAG1, SOX10, and Myb protein expression in various salivary gland neoplasm tissues.
Methods
A total of 113 cases of surgically resected salivary gland neoplasms at the NationalCancer Center from January 2007 to March 2017 were identified. Immunohistochemical stainingof PLAG1, SOX10, and Myb in tissue samples was performed using tissue microarrays.
Results
Among the 113 cases, 82 (72.6%) were benign and 31 (27.4%) were malignant. PLAG1 showednuclear staining and normal parotid gland was not stained. Among 48 cases of pleomorphicadenoma, 29 (60.4%) were positive for PLAG1. All other benign and malignant salivary glandneoplasms were PLAG1-negative. SOX10 showed nuclear staining. In normal salivary gland tissuesSOX10 was expressed in cells of acinus and intercalated ducts. In benign tumors, SOX10 expressionwas observed in all pleomorphic adenoma (48/48), and basal cell adenoma (3/3), but not inother benign tumors. SOX10 positivity was observed in nine of 31 (29.0%) malignant tumors.Myb showed nuclear staining but was not detected in normal parotid glands. Four of 31 (12.9%)malignant tumors showed Myb positivity: three adenoid cystic carcinomas (AdCC) and onemyoepithelial carcinoma with focal AdCC-like histology.
Conclusions
PLAG1 expression is specificto pleomorphic adenoma. SOX10 expression is helpful to rule out excretory duct origin tumor,but its diagnostic value is relatively low. Myb is useful for diagnosing AdCC when histology isunclear in the surgical specimen. -
Citations
Citations to this article as recorded by- Pleomorphic adenoma gene 1 (PLAG1) protects p53-/- myoepithelial cells from mitochondria-related apoptosis caused by hypoxia
Nodoka Kindaichi, Yoshiki Mukudai, Yuzo Abe, Masataka Watanabe, Maki Nara, Konomi Yamada, Asami Houri, Toshikazu Shimane, Tatsuo Shirota
Journal of Oral and Maxillofacial Surgery, Medicine, and Pathology.2025; 37(4): 654. CrossRef - Retrospective Clinicopathological Study of 33 Cases of Pleomorphic Salivary Adenoma Diagnosed in Benghazi
Siraj S. Najem, Elhoni Ashour, Rehab Elmaddani, Ali M. Elmurtadi
Libyan Journal of Dentistry .2025; 8(2): 29. CrossRef - Pleomorphic adenoma of palatal minor salivary glands
Afrah Aldelaimi, Tahrir Aldelaimi, Suzan Abdulkareem
Revista Española de Cirugía Oral y Maxilofacial.2025;[Epub] CrossRef - Immunohistochemical Characterization of a Large Cohort of Triple Negative Breast Cancer
Rachel Han, Sharon Nofech-Mozes, Dina Boles, Hannah Wu, Nikolina Curcin, Elzbieta Slodkowska
International Journal of Surgical Pathology.2024; 32(2): 239. CrossRef - Proceedings of the 2024 North American Society of Head and Neck Pathology Companion Meeting, Baltimore, MD, March 24, 2024: Navigating Ancillary Studies in Basaloid/Blue Salivary Tumors
Kristine S. Wong
Head and Neck Pathology.2024;[Epub] CrossRef - Insights into the molecular alterations of PLAG1 and HMGA2 associated with malignant phenotype acquisition in pleomorphic adenoma
Reydson Alcides de Lima-Souza, Gustavo de Souza Vieira, Talita de Carvalho Kimura, João Figueira Scarini, Luccas Lavareze, Tayná Figueiredo Maciel, Moisés Willian Aparecido Gonçalves, Erika Said Abu Egal, Albina Altemani, Fernanda Viviane Mariano
Critical Reviews in Oncology/Hematology.2024; 204: 104494. CrossRef - Expanding the Molecular Spectrum of Carcinoma Ex Pleomorphic Adenoma
Reydson Alcides de Lima-Souza, Albina Altemani, Michal Michal, Fernanda Viviane Mariano, Ilmo Leivo, Alena Skálová
American Journal of Surgical Pathology.2024; 48(12): 1491. CrossRef - Utility of SOX10 and estrogen receptor immunohistochemical expression in endometrial carcinoma of Egyptian patients
Mona A. Kora, Alyaa A. Moselhy, Rania A. Abdallah
Egyptian Journal of Pathology.2024; 44(2): 190. CrossRef - Exploring Advanced Diagnostic Techniques for Salivary Gland Disorders: A Narrative Overview
Chuan-Xiang Li-, Liu Zhang, Ya-Ru Yan, Yong-Jie Ding, Ying-Ni Lin, Jian-Ping Zhou, Ning Li, Hong-Peng Li, Shi-Qi Li, Xian-Wen Sun, Qing-Yun Li
Asian journal of Current Research in Clinical Cancer.2024; 4(1): 1. CrossRef - The Challenge of “Monomorphic” Mucoepidermoid Carcinoma—Report of a Rare Case with Pure Spindle-Clear Cell Morphology
Xinyi Qu, Edwin Jun Chen Chew, Sathiyamoorthy Selvarajan, Bingcheng Wu, Abbas Agaimy, Fredrik Petersson
Head and Neck Pathology.2023; 17(3): 864. CrossRef - SOX10
Albert L Sy, Mai P Hoang
Journal of Clinical Pathology.2023; 76(10): 649. CrossRef - Activating Transcription Factor 1 (ATF1) Immunohistochemical Marker Distinguishes HCCC from MEC
Wafaey Badawy, Asmaa S. Abdelfattah, Haneen A. Sallam
Journal of Molecular Pathology.2023; 4(3): 178. CrossRef - Rare case of pleomorphic adenoma presenting as peritonsilar tumor
Anđelina Jovanović, Svetlana Valjarević, Milan Jovanović
Medicinska istrazivanja.2023; 56(3): 95. CrossRef - Pleomorphic Adenoma of a Minor Salivary Gland of the Hard Palate: A Case Report
Ishank Panchal, Anil Wanjari
Cureus.2023;[Epub] CrossRef - Advanced Diagnostic Methods for Salivary Glands Diseases: A Narrative Review Study
Malak Mohammed AlOsaimi, Abdulaziz Mohammed AlSubaheen, Taif Saleh Jameel, Rand Abdulrahman AlSalamah, Dalal Naseh AlAnzi, Norah Ameen AlOushan, Fahad Fadhel AlShammari, Cristalle Soman
Clinical Cancer Investigation Journal.2023; 12(4): 19. CrossRef - Clinical Significance of SOX10 Expression in Human Pathology
Hisham F. Bahmad, Aran Thiravialingam, Karthik Sriganeshan, Jeffrey Gonzalez, Veronica Alvarez, Stephanie Ocejo, Alvaro R. Abreu, Rima Avellan, Alejandro H. Arzola, Sana Hachem, Robert Poppiti
Current Issues in Molecular Biology.2023; 45(12): 10131. CrossRef - NR4A3 Immunostain Is a Highly Sensitive and Specific Marker for Acinic Cell Carcinoma in Cytologic and Surgical Specimens
Kartik Viswanathan, Shaham Beg, Bing He, Taotao Zhang, Richard Cantley, Daniel J Lubin, Qiuying Shi, Zahra Maleki, Saeed Asiry, Rema Rao, Nora Katabi, Masato Nakaguro, William C Faquin, Peter M Sadow, Momin T Siddiqui, Theresa Scognamiglio
American Journal of Clinical Pathology.2022; 157(1): 98. CrossRef - Recent Advances on Immunohistochemistry and Molecular Biology for the Diagnosis of Adnexal Sweat Gland Tumors
Nicolas Macagno, Pierre Sohier, Thibault Kervarrec, Daniel Pissaloux, Marie-Laure Jullie, Bernard Cribier, Maxime Battistella
Cancers.2022; 14(3): 476. CrossRef - Diagnostic accuracy of human transcriptional activator (Myb) expression by ELISA technique versus immunohistochemistry in detecting salivary gland carcinomas
Yousra Refaey, OlfatGamil Shaker, Ayman Abdelwahab, ImanAdel Mohamed Abdelmoneim, Fat’heyaMohamed Zahran
Journal of International Oral Health.2022; 14(1): 61. CrossRef - SLUG is a key regulator of epithelial-mesenchymal transition in pleomorphic adenoma
Hyesung Kim, Seung Bum Lee, Jae Kyung Myung, Jeong Hwan Park, Eunsun Park, Dong Il Kim, Cheol Lee, Younghoon Kim, Chul-Min Park, Min Bum Kim, Gil Chai Lim, Bogun Jang
Laboratory Investigation.2022; 102(6): 631. CrossRef - Assessment of MEF2C as a novel myoepithelial marker using normal salivary gland and pleomorphic adenoma: An immunohistochemical study
Ikuko Takakura, Satoko Kujiraoka, Rika Yasuhara, Junichi Tanaka, Fumio Ide, Kenji Mishima
Journal of Oral and Maxillofacial Surgery, Medicine, and Pathology.2022; 34(4): 523. CrossRef - Update on selective special types of breast neoplasms: Focusing on controversies, differential diagnosis, and molecular genetic advances
Shi Wei
Seminars in Diagnostic Pathology.2022; 39(5): 367. CrossRef - Cutaneous Melanomas: A Single Center Experience on the Usage of Immunohistochemistry Applied for the Diagnosis
Costantino Ricci, Emi Dika, Francesca Ambrosi, Martina Lambertini, Giulia Veronesi, Corti Barbara
International Journal of Molecular Sciences.2022; 23(11): 5911. CrossRef - Distinct clinicopathological and genomic features in solid and basaloid adenoid cystic carcinoma of the breast
Juan Ji, Fang Zhang, Fanglei Duan, Hong Yang, Jun Hou, Yang Liu, Jie Dai, Qiong Liao, Xian Chen, Qingsong Liu
Scientific Reports.2022;[Epub] CrossRef - NR4A3 fluorescence in situ hybridization analysis in cytologic and surgical specimens of acinic cell carcinoma
Qiuying Shi, Bin Zhang, Caroline Bsirini, Liqiong Li, Ellen J. Giampoli, Kelly R. Magliocca, Michelle Reid, Zhongren Zhou
Human Pathology.2022; 127: 86. CrossRef - Evaluation of NR4A3 immunohistochemistry (IHC) and fluorescence in situ hybridization and comparison with DOG1 IHC for FNA diagnosis of acinic cell carcinoma
John M. Skaugen, Raja R. Seethala, Simion I. Chiosea, Michael S. Landau
Cancer Cytopathology.2021; 129(2): 104. CrossRef -
MYB-NFIB Translocation by FISH in Adenoid Cystic Carcinoma of the Head and Neck in Nigerian Patients: A Preliminary Report
Adepitan A. Owosho, Olufunlola M. Adesina, Oluwole Odujoko, Olujide O. Soyele, Akinwumi Komolafe, Robert Bauer, Kallie Holte, Kurt F. Summersgill
Head and Neck Pathology.2021; 15(2): 433. CrossRef - Liquid-based cytology of oral brushings in a case of adenoid cystic carcinoma arising from the palate
Ryo MAKINO, Akihiko KAWAHARA, Hideyuki ABE, Yorihiko TAKASE, Chihiro FUKUMITSU, Kazuya MURATA, Tomoko YOSHIDA, Yukako SHINODA, Yoshiki NAITO, Jun AKIBA
The Journal of the Japanese Society of Clinical Cytology.2021; 60(1): 33. CrossRef - MYB Translocations in Both Myoepithelial and Ductoglandular Epithelial Cells in Adenoid Cystic Carcinoma: A Histopathologic and Genetic Reappraisal in Six Primary Cutaneous Cases
Keisuke Goto, Kazuyoshi Kajimoto, Takashi Sugino, Shin-ichi Nakatsuka, Makoto Yoshida, Mai Noto, Michihiro Kono, Toshihiro Takai
The American Journal of Dermatopathology.2021; 43(4): 278. CrossRef - Co-expression of Myoepithelial and Melanocytic Features in Carcinoma Ex Pleomorphic Adenoma
Costantino Ricci, Federico Chiarucci, Francesca Ambrosi, Tiziana Balbi, Barbara Corti, Ottavio Piccin, Ernesto Pasquini, Maria Pia Foschini
Head and Neck Pathology.2021; 15(4): 1385. CrossRef - Juvenile onset pleomorphic adenoma presenting as giant tumor of parotid gland in a young female
Surender Verma, Shivika Aggarwal, Pradeep Garg, Anjali Verma, Mridul Gera, Swaran S. Yadav
Journal of Dr. NTR University of Health Sciences.2021; 10(4): 286. CrossRef - Cytopathology and diagnostics of Warthin's tumour
Mirna Sučić, Nives Ljubić, Leila Perković, Dunja Ivanović, Leo Pažanin, Tena Sučić Radovanović, Dubravka Župnić‐Krmek, Fabijan Knežević
Cytopathology.2020; 31(3): 193. CrossRef - Clear cell papillary neoplasm of the breast with MAML2 gene rearrangement: Clear cell hidradenoma or low-grade mucoepidermoid carcinoma?
Raima A. Memon, Carlos N Prieto Granada, Shi Wei
Pathology - Research and Practice.2020; 216(10): 153140. CrossRef
- Pleomorphic adenoma gene 1 (PLAG1) protects p53-/- myoepithelial cells from mitochondria-related apoptosis caused by hypoxia
- Fine-Needle Aspiration Cytology of Carcinosarcoma in the Salivary Gland: An Extremely Rare Case Report
- Hyo Jung An, Hye Jin Baek, Jin Pyeong Kim, Min Hye Kim, Dae Hyun Song
- J Pathol Transl Med. 2018;52(2):136-139. Published online December 28, 2017
- DOI: https://doi.org/10.4132/jptm.2017.07.27
- 8,126 View
- 135 Download
- 5 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF - Carcinosarcoma of the salivary gland is an extremely rare tumor that is composed of both malignant epithelial and mesenchymal components. Diagnosing carcinosarcoma with fine-needle aspiration cytology is challenging because of its overlapping cytomorphologic characteristics with other high-grade malignant salivary gland tumors. Among the many features, including pleomorphic oncocytoid epithelial components, necrotic background, and mitoses, recognizing the singly scattered atypical spindle cells is most essential in carcinosarcoma. We present a case of a 66-year-old male patient with characteristic features of carcinosarcoma, who was successfully treated by wide local excision and subsequent radiation therapy.
-
Citations
Citations to this article as recorded by- A Rare Osteoid Forming Carcinosarcoma Ex‐Pleomorphic Adenoma of the Parotid Gland
Nyein Nyein Htun, Daniel Nguyen, Beverly Y. Wang, Anoosh Montaser, Behdokht Nowroozizadeh, Suraiya Saleem
Case Reports in Pathology.2025;[Epub] CrossRef - Carcinosarcoma of the parotid gland: a case report and review of the literature
Swachi Jain, Mohammed Abdelwahed, Daniel Hector Chavarria, Lucio Pereira, Gary Stone, Alan Johnson, Jian Yi Li
Journal of Medical Case Reports.2024;[Epub] CrossRef - Is Primary Poorly Differentiated Sarcomatoid Malignancy of the Parotid Gland Sarcomatoid Undifferentiated/Dedifferentiated Melanoma? Report of Three Unusual Cases Diagnosed by Fine-Needle Aspiration Combined with Histological, Immunohistochemical, and Mol
Jerzy Klijanienko, Julien Masliah-Planchon, Olivier Choussy, Guillaume Rougier, Antoine Dubray Vautrin, Maria Lesnik, Nathalie Badois, Wahib Ghanem, Jan Klos, Christophe Le Tourneau, Gregoire Marret, Raymond Barnhill, Adel K. El-Naggar
Acta Cytologica.2024; 68(2): 107. CrossRef - Carcinosarcoma of the deep lobe of the parotid gland in the parapharyngeal region: A case report
Yue-Yang Tang, Gui-Quan Zhu, Zhi-Jian Zheng, Li-Hong Yao, Zi-Xin Wan, Xin-Hua Liang, Ya-Ling Tang
World Journal of Clinical Cases.2023; 11(31): 7663. CrossRef - Carcinosarcoma of Submandibular Salivary Gland with a Rare Sarcomatous Variant
Shalini Bhalla, Naseem Akhtar, Puneet Prakash, Malti Kumari, Madhu Mati Goel
Indian Journal of Surgical Oncology.2019; 10(1): 61. CrossRef
- A Rare Osteoid Forming Carcinosarcoma Ex‐Pleomorphic Adenoma of the Parotid Gland
- Accuracy of Core Needle Biopsy Versus Fine Needle Aspiration Cytology for Diagnosing Salivary Gland Tumors
- In Hye Song, Joon Seon Song, Chang Ohk Sung, Jong-Lyel Roh, Seung-Ho Choi, Soon Yuhl Nam, Sang Yoon Kim, Jeong Hyun Lee, Jung Hwan Baek, Kyung-Ja Cho
- J Pathol Transl Med. 2015;49(2):136-143. Published online March 12, 2015
- DOI: https://doi.org/10.4132/jptm.2015.01.03
- 17,412 View
- 238 Download
- 79 Web of Science
- 82 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Core needle biopsy is a relatively new technique used to diagnose salivary gland lesions, and its role in comparison with fine needle aspiration cytology needs to be refined. Methods: We compared the results of 228 ultrasound-guided core needle biopsy and 371 fine needle aspiration procedures performed on major salivary gland tumors with their postoperative histological diagnoses. Results: Core needle biopsy resulted in significantly higher sensitivity and more accurate tumor subtyping, especially for malignant tumors, than fine needle aspiration. No patient developed major complications after core needle biopsy. Conclusions: We recommend ultrasoundguided core needle biopsy as the primary diagnostic tool for the preoperative evaluation of patients with salivary gland lesions, especially when malignancy is suspected. -
Citations
Citations to this article as recorded by- Fine-needle aspiration or core needle biopsy? A meta-analysis of diagnostic accuracy and procedural outcomes in salivary gland tumors
Kenan Kassem, Alaa Safia, Uday Abd Elhadi, Shlomo Merchavy
European Journal of Radiology.2026; 194: 112532. CrossRef - Controversial issues in core needle biopsy for diagnosing parotid neoplasms
N. V. Vishneva, P. A. Demenchuk, O. Yu. Petropavlovskaya, E. I. Selifanova, A. N. Lanina, N. V. Kalakutsky, A. I. Yaremenko
Parodontologiya.2026;[Epub] CrossRef - Frozen Section Analysis in Submandibular Gland Tumors: Optimizing Intraoperative Decision-Making
Amir Bolooki, Felix Johnson, Anna Stenzl, Zhaojun Zhu, Benedikt Gabriel Hofauer
Cancers.2025; 17(5): 895. CrossRef - The Myriad Spectrum of Salivary Gland Lesions: Cytohistological Correlation on Fine Needle Aspiration Cytology, Core Needle Biopsy, and Resections in a 5‐Year Single Institutional Experience of North India
Zachariah Chowdhury, Pallavi Majumdar, Sumeet Narain, Komal Lamba
Diagnostic Cytopathology.2025; 53(8): 391. CrossRef - Salivary Duct Carcinoma: A 12-Year Single Center Experience
Hyowon Ahn, Dongbin Ahn, Ji Hye Kwak
Korean Journal of Otorhinolaryngology-Head and Neck Surgery.2025; 68(6): 232. CrossRef - Cytological Spectrum of Salivary Gland Lesions Using the Milan System and their Histopathological Correlation: Retrospective Study in a Peripheral Medical College of Eastern India
Ujjwal Bandyopadhyay, Sarmila Guha Banerjee, Sirshak Dutta
Indian Journal of Otolaryngology and Head & Neck Surgery.2025; 77(11): 4887. CrossRef - Parotid Secretory Carcinoma: A Distinct Entity from Acinic Cell Carcinoma
Muhammad Adzha Musa, Muhamad Arif Ahmad Radzi, Moharzudi Mohamed, Ikram Hakim
Indian Journal of Otolaryngology and Head & Neck Surgery.2025;[Epub] CrossRef - Diagnostic performance of ultrasound guided salivary gland core needle biopsy and fine needle aspiration in children
Vicente Oliveira, Anthea Lafrenière, Nikolaus Wolter, Joao Amaral, Alessandro Gasparetto, Dimitri Parra Rojas
Pediatric Radiology.2025;[Epub] CrossRef - Giant Pleomorphic Adenoma of Submandibular Gland
Harendra Kumar, Qazi Saquib Rizwan, Mayank Gupta, Tarun Kumar
Indian Journal of Otolaryngology and Head & Neck Surgery.2024; 76(1): 1361. CrossRef - CT-guided core needle biopsies of head and neck tumors: a comprehensive monocenter analysis of safety and outcomes
Thomas Joseph Vogl, Heinrich Johannes Ketelsen, Scherwin Mahmoudi, Jan-Erik Scholtz, Vitali Koch, Leon David Grünewald, Peter Wild, Timo Stoever, Simon Bernatz
European Radiology.2024; 34(8): 5370. CrossRef - Indications for Submandibulectomy Within a 20-Year Period
Amir Bolooki, Anna Stenzl, Christopher Weusthof, Benedikt Hofauer
Ear, Nose & Throat Journal.2024;[Epub] CrossRef - Treatment of Warthin’s Tumors of the Parotid Gland With Radiofrequency Ablation: A Systematic Review of the Current Literature
Kenny Do, Eric Kawana, Sisi Tian, Jo-Lawrence Bigcas
Ear, Nose & Throat Journal.2024;[Epub] CrossRef - Evaluation of the anterior processes of the parotid gland: an ultrasonographic study
Tarık Ali Uğur, Hümeyra Tercanlı
Surgical and Radiologic Anatomy.2024; 46(6): 915. CrossRef - Salivary Gland Fine-Needle Aspiration
Federica Policardo, Antonino Mule’, Esther Diana Rossi
Surgical Pathology Clinics.2024; 17(3): 347. CrossRef - Implementation of the Milan System for Reporting Salivary Gland Cytology: A Two-Year Outcome Cytopathology Data of a Tertiary Care Center
Soudamini Mahapatra, Chinmaya Sundar Ray, Aparajita Mishra, Dileswari Pradhan
Cureus.2024;[Epub] CrossRef - Preoperative cytopathological investigatory aids in the diagnosis of salivary gland lesions
S Vidyalakshmi, K Shanmugasamy
Journal of Oral and Maxillofacial Pathology.2024; 28(2): 172. CrossRef - Machine Learning on Ultrasound Texture Analysis Data for Characterizing of Salivary Glandular Tumors: A Feasibility Study
Li-Jen Liao, Ping-Chia Cheng, Feng-Tsan Chan
Diagnostics.2024; 14(16): 1761. CrossRef - Association of Membranous Basal Cell Adenoma and Basal Cell Adenocarcinoma With Brooke-Spiegler Syndrome
Lexi Goehring, Debby Rampisela, Jordan L Pleitz
Cureus.2024;[Epub] CrossRef - A Retrospective 8‐Year Single Institutional Study in Germany Regarding Diagnosis, Treatment, and Outcome of Malignant Parotid Tumors
S. Andrianopoulou, L. S. Fiedler, B. M. Lippert, O. C. Bulut, Mohamed Rahouma
International Journal of Surgical Oncology.2024;[Epub] CrossRef - High Field MRI in Parotid Gland Tumors: A Diagnostic Algorithm
Chiara Gaudino, Andrea Cassoni, Martina Lucia Pisciotti, Resi Pucci, Chiara Veneroso, Cira Rosaria Tiziana Di Gioia, Francesca De Felice, Patrizia Pantano, Valentino Valentini
Cancers.2024; 17(1): 71. CrossRef - Nationwide Incidence Trends of Pediatric Parotid Malignancy in Korea and a Retrospective Analysis of Single-Institution Surgical Experience of Parotidectomy
Hyun Seong Kim, Seo Young Kim, Eun-Jae Chung, Seong Keun Kwon, Soon-Hyun Ahn, Yuh-Seog Jung, Jungirl Seok
Korean Society of Head and Neck Oncology.2024; 40(2): 7. CrossRef - The Usefulness of Ultrasound-Guided Core Needle Biopsy Compared to Fine Needle Aspiration in Pre-Operative Diagnosis of Cystic-Predominant Parotid Tumors
Youn Jin Cho, Young Rok Jo, Hyun Jun Hong, Hye Ran Lee
Korean Journal of Otorhinolaryngology-Head and Neck Surgery.2023; 66(8): 532. CrossRef - The Value of Ultrasound-guided Core Needle Biopsy in Differentiating Benign from Malignant Salivary Gland Lesions
Mohammad Ali Kazemi, Farzaneh Amini, Bita Kargar, Maryam Lotfi, Keyvan Aghazadeh, Hashem Sharifian, Behnaz Moradi, Javid Azadbakht
Indian Journal of Otolaryngology and Head & Neck Surgery.2023; 75(2): 266. CrossRef - Schnellschnittdiagnostik bei Tumoren des Trigonum submandibulare
S. Riemann, A. Knopf
HNO.2023; 71(3): 164. CrossRef - Myoepithelial Carcinoma Ex Pleomorphic Adenoma of the Submandibular Gland: A Case Report
Georgia Syrnioti, Antonia Syrnioti, Alharith Abdullah, Xuehui Lui, Ernesto Mendoza
Cureus.2023;[Epub] CrossRef - Intraductal Carcinoma: The Carcinoma In Situ of the Salivary Gland
Rhema Thomas, Tijjani Umar, Farzad Borumandi
Journal of Craniofacial Surgery.2023; 34(5): e432. CrossRef - Fine-Needle Aspiration Cytology for Parotid Tumors
Masataka Taniuchi, Tetsuya Terada, Ryo Kawata
Life.2022; 12(11): 1897. CrossRef - Utility of the Milan System for Reporting Salivary Gland Cytology, with focus on the incidence and histologic correlates of atypia of undetermined significance (AUS) and salivary gland neoplasm of uncertain malignant potential (SUMP): A 3‐year institution
Christopher M. Cormier, Shweta Agarwal
Cancer Cytopathology.2022; 130(4): 303. CrossRef - Percutaneous CT-Guided Core Needle Biopsies of Head and Neck Masses: Review of 184 Cases at a Single Academic Institution, Common and Special Techniques, Diagnostic Yield, and Safety
R.W. Jordan, D.P. Shlapak, J.C. Benson, F.E. Diehn, D.K. Kim, V.T. Lehman, G.B. Liebo, A.A. Madhavan, J.M. Morris, P.P. Morris, J.T. Verdoorn, C.M. Carr
American Journal of Neuroradiology.2022; 43(1): 117. CrossRef - Nodular fasciitis of the submandibular gland
Ting Suen Wong, Richard Wei Chern Gan, Laszlo Karsai, Bun Yin Winson Wong
BMJ Case Reports.2022; 15(4): e245584. CrossRef - Validation of the Milan system for reporting salivary gland cytopathology: a single institution’s 10-year experience
Christopher Felicelli, Joseph Reznicek, Yevgen Chornenkyy, Lucy Jager, Daniel Johnson
Journal of the American Society of Cytopathology.2022; 11(5): 264. CrossRef - Application of the Milan system for reporting salivary gland cytopathology using cell blocks
Grégoire B. Morand, Raihanah Alsayegh, Alex M. Mlynarek, Marianne Plourde, Tiffany Mach, Marco A. Mascarella, Michael P. Hier, Livia Florianova, Marc P. Pusztaszeri
Virchows Archiv.2022; 481(4): 575. CrossRef - Comparisons among the Ultrasonography Prediction Model, Real-Time and Shear Wave Elastography in the Evaluation of Major Salivary Gland Tumors
Ping-Chia Cheng, Wu-Chia Lo, Chih-Ming Chang, Ming-Hsun Wen, Po-Wen Cheng, Li-Jen Liao
Diagnostics.2022; 12(10): 2488. CrossRef - A Novel Sonographic Scoring Model in the Prediction of Major Salivary Gland Tumors
Wu‐Chia Lo, Chih‐Ming Chang, Chi‐Te Wang, Po‐Wen Cheng, Li‐Jen Liao
The Laryngoscope.2021;[Epub] CrossRef - Assessing the diagnostic accuracy for pleomorphic adenoma and Warthin tumor by employing the Milan System for Reporting Salivary Gland Cytopathology: An international, multi‐institutional study
Derek B. Allison, Alexander P. Smith, Daniel An, James Adam Miller, Khurram Shafique, Sharon Song, Kartik Viswanathan, Elizabeth Eykman, Rema A. Rao, Austin Wiles, Güliz A. Barkan, Ritu Nayar, Guido Fadda, Celeste N. Powers, Esther Diana Rossi, Momin T. S
Cancer Cytopathology.2021; 129(1): 43. CrossRef - Magnetic resonance imaging of salivary gland tumours: Key findings for imaging characterisation
Davide Maraghelli, Michele Pietragalla, Cesare Cordopatri, Cosimo Nardi, Anna Julie Peired, Giandomenico Maggiore, Stefano Colagrande
European Journal of Radiology.2021; 139: 109716. CrossRef - The Milan System, from Its Introduction to Its Current Adoption in the Diagnosis of Salivary Gland Cytology
Esther Diana Rossi
Journal of Molecular Pathology.2021; 2(2): 114. CrossRef - Utility of the Milan system for reporting salivary gland cytopathology during rapid on‐site evaluation (ROSE) of salivary gland aspirates
Aanchal Kakkar, Mukin Kumar, Priyadarsani Subramanian, Arshad Zubair, Rajeev Kumar, Alok Thakar, Deepali Jain, Sandeep R. Mathur, Venkateswaran K. Iyer
Cytopathology.2021; 32(6): 779. CrossRef - Contribution of small tissue biopsy and flow cytometry to preoperative cytological categorization of salivary gland fine needle aspirates according to the Milan System: Single center experience on 287 cases
Tolga Bağlan, Serpil Dizbay Sak, Cevriye Cansız Ersöz, Koray Ceyhan
Diagnostic Cytopathology.2021; 49(4): 509. CrossRef - Is Milan for kids?: The Milan System for Reporting Salivary Gland Cytology in pediatric patients at an academic children's hospital with cytologic‐histologic correlation
Swati P. Satturwar, Maren Y. Fuller, Sara E. Monaco
Cancer Cytopathology.2021; 129(11): 884. CrossRef - Radiographic Interpretation in Oral Medicine and Hospital Dental Practice
Katherine France, Anwar A.A.Y. AlMuzaini, Mel Mupparapu
Dental Clinics of North America.2021; 65(3): 509. CrossRef - Carcinoma ex pleomorphic adenoma of major salivary glands: CT and MR imaging findings
Can Wang, Qiang Yu, Siyi Li, Jingjing Sun, Ling Zhu, Pingzhong Wang
Dentomaxillofacial Radiology.2021; 50(7): 20200485. CrossRef - Salivary gland carcinoma in children and adolescents: The EXPeRT/PARTNER diagnosis and treatment recommendations
Aurore Surun, Dominik T. Schneider, Andrea Ferrari, Teresa Stachowicz‐Stencel, Jelena Rascon, Anna Synakiewicz, Abbas Agaimy, Kata Martinova, Denis Kachanov, Jelena Roganovic, Ewa Bien, Gianni Bisogno, Ines B. Brecht, Frédéric Kolb, Juliette Thariat, Anto
Pediatric Blood & Cancer.2021;[Epub] CrossRef - A Call for Universal Acceptance of the Milan System for Reporting Salivary Gland Cytopathology
Eric Barbarite, Sidharth V. Puram, Adeeb Derakhshan, Esther D. Rossi, William C. Faquin, Mark A. Varvares
The Laryngoscope.2020; 130(1): 80. CrossRef - Preoperative biopsy in parotid malignancies: Variation in use and impact on surgical margins
Liliya Benchetrit, Sina J. Torabi, Elliot Morse, Saral Mehra, Rahmatullah Rahmati, Heather A. Osborn, Benjamin L. Judson
The Laryngoscope.2020; 130(6): 1450. CrossRef - α‐Synuclein Real‐Time Quaking‐Induced Conversion in the Submandibular Glands of Parkinson's Disease Patients
Sireesha Manne, Naveen Kondru, Huajun Jin, Vellareddy Anantharam, Xuemei Huang, Arthi Kanthasamy, Anumantha G. Kanthasamy
Movement Disorders.2020; 35(2): 268. CrossRef - The Accessory Parotid Gland and its Clinical Significance
Mateusz A. Rosa, Dominik P. Łazarz, Jakub R. Pękala, Bendik Skinningsrud, Sigurd S. Lauritzen, Bernard Solewski, Przemysław A. Pękala, Jerzy A. Walocha, Krzysztof A. Tomaszewski
Journal of Craniofacial Surgery.2020; 31(3): 856. CrossRef - Comparison of core needle biopsy and fine‐needle aspiration in diagnosis of ma lignant salivary gland neoplasm: Systematic review and meta‐analysis
Jungheum Cho, Junghoon Kim, Ji Sung Lee, Choong Guen Chee, Youngjune Kim, Sang Il Choi
Head & Neck.2020; 42(10): 3041. CrossRef - The Milan system for reporting salivary gland cytopathology: The clinical impact so far. Considerations from theory to practice
Esther Diana Rossi, William C. Faquin
Cytopathology.2020; 31(3): 181. CrossRef - The role of core needle biopsy in parotid glands lesions with inconclusive fine needle aspiration
Farrokh Heidari, Firouzeh Heidari, Benyamin Rahmaty, Neda Jafari, Kayvan Aghazadeh, Saeed Sohrabpour, Ebrahim Karimi
American Journal of Otolaryngology.2020; 41(6): 102718. CrossRef - Role of Fine Needle Aspiration Cytology in the Diagnosis of Parotid Gland Tumors: Analysis of 193 Cases
Rahim Dhanani, Haissan Iftikhar, Muhammad Sohail Awan, Nida Zahid, Sehrish Nizar Ali Momin
International Archives of Otorhinolaryngology.2020; 24(04): e508. CrossRef - Cytohistological correlation of salivary gland tumours with emphasis on Milan system for reporting: A novel step towards internal quality assurance
Anandraj Vaithy.K, ATM Venkat Raghava, E S Keerthika Sri, K R Umadevi
IP Archives of Cytology and Histopathology Research.2020; 5(4): 283. CrossRef - Diagnosing Recently Defined and Uncommon Salivary Gland Lesions in Limited Cellularity Specimens: Cytomorphology and Ancillary Studies
Esther Diana Rossi, Zubair Baloch, William Faquin, Liron Pantanowitz
AJSP: Reviews and Reports.2020; 25(5): 210. CrossRef - Peripheral T Cell Lymphoma of Parotid Gland: A Diagnostic Challenge
J. G. Aishwarya, Satish Nair, C. N. Patil, Swarna Shivakumar, N. Shrivalli, Ashish Shah
Indian Journal of Otolaryngology and Head & Neck Surgery.2019; 71(S1): 533. CrossRef - Potential utility of core needle biopsy in the diagnosis of IgG4-related dacryoadenitis and sialadenitis
Kenichi Takano, Tsuyoshi Okuni, Keisuke Yamamoto, Ryuta Kamekura, Ryoto Yajima, Motohisa Yamamoto, Hiroki Takahashi, Tetsuo Himi
Modern Rheumatology.2019; 29(2): 393. CrossRef - Retrospective assessment of the effectiveness of the Milan system for reporting salivary gland cytology: A systematic review and meta‐analysis of published literature
Sahar J Farahani, Zubair Baloch
Diagnostic Cytopathology.2019; 47(2): 67. CrossRef - Values of fine‐needle aspiration cytology of parotid gland tumors: A review of 996 cases at a single institution
Manabu Suzuki, Ryo Kawata, Masaaki Higashino, Shuji Nishikawa, Tetsuya Terada, Shin‐Ichi Haginomori, Yoshitaka Kurisu, Yoshinobu Hirose
Head & Neck.2019; 41(2): 358. CrossRef - Positive Surgical Margins in Submandibular Malignancies: Facility and Practice Variation
Liliya Benchetrit, Elliot Morse, Benjamin L. Judson, Saral Mehra
Otolaryngology–Head and Neck Surgery.2019; 161(4): 620. CrossRef - The growth rate and the positive prediction of needle biopsy of clinically diagnosed Warthin’s tumor
Jungirl Seok, Woo-Jin Jeong, Soon-Hyun Ahn, Young Ho Jung
European Archives of Oto-Rhino-Laryngology.2019; 276(7): 2091. CrossRef - The Difference in the Clinical Features Between Carcinoma ex Pleomorphic Adenoma and Pleomorphic Adenoma
Jungirl Seok, Se Jin Hyun, Woo-Jin Jeong, Soon-Hyun Ahn, Hyojin Kim, Young Ho Jung
Ear, Nose & Throat Journal.2019; 98(8): 504. CrossRef - Fine‐needle aspiration cytology and radiological imaging in parotid gland tumours: Our experience in 103 patients
Clare Perkins, Edward Toll, Philip Reece
Clinical Otolaryngology.2019; 44(6): 1124. CrossRef - Application of the Milan system of reporting salivary cytopathology – A retrospective cytohistological correlation study
Ramya Katta, Devi Padmavathi Chaganti
Journal of Dr. NTR University of Health Sciences.2019; 8(1): 11. CrossRef - Ultrasound‐guided core needle biopsy in salivary glands: A meta‐analysis
Hee Joon Kim, Jong Seung Kim
The Laryngoscope.2018; 128(1): 118. CrossRef - Accuracy and effectiveness of ultrasound-guided core-needle biopsy in the diagnosis of focal lesions in the salivary glands
Jose Luis del Cura, Gloria Coronado, Rosa Zabala, Igone Korta, Ignacio López
European Radiology.2018; 28(7): 2934. CrossRef - The Milan System for Reporting Salivary Gland Cytopathology (MSRSGC): an ASC-IAC–sponsored system for reporting salivary gland fine-needle aspiration
Esther Diana Rossi, Zubair Baloch, Marc Pusztaszeri, William C. Faquin
Journal of the American Society of Cytopathology.2018; 7(3): 111. CrossRef - Routine and Advanced Ultrasound of Major Salivary Glands
Kunwar Suryaveer Singh Bhatia, Yuk-Ling Dai
Neuroimaging Clinics of North America.2018; 28(2): 273. CrossRef - The Milan System for Reporting Salivary Gland Cytopathology (MSRSGC): An ASC-IAC-Sponsored System for Reporting Salivary Gland Fine-Needle Aspiration
Esther Diana Rossi, Zubair W. Baloch, Marc Pusztaszeri, William C. Faquin
Acta Cytologica.2018; 62(3): 157. CrossRef - Evaluation and diagnosis of salivary gland neoplasms
Erica Jackson Mayland, Anna M. Pou
Operative Techniques in Otolaryngology-Head and Neck Surgery.2018; 29(3): 129. CrossRef - Feasibility and Safety of Multicenter Tissue and Biofluid Sampling for α-Synuclein in Parkinson’s Disease: The Systemic Synuclein Sampling Study (S4)
Lana M. Chahine, Thomas G. Beach, Nicholas Seedorff, Chelsea Caspell-Garcia, Christopher S. Coffey, Michael Brumm, Charles H. Adler, Geidy E. Serrano, Carly Linder, Sherri Mosovsky, Tatiana Foroud, Holly Riss, Dixie Ecklund, John Seibyl, Danna Jennings, V
Journal of Parkinson’s Disease.2018; 8(4): 517. CrossRef - Preoperative diagnostic of parotid gland neoplasms: fine-needle aspiration cytology or core needle biopsy?
Peter Zbären, Asterios Triantafyllou, Kenneth O. Devaney, Vincent Vander Poorten, Henrik Hellquist, Alessandra Rinaldo, Alfio Ferlito
European Archives of Oto-Rhino-Laryngology.2018; 275(11): 2609. CrossRef - A comparison study of the reporting systems for salivary gland fine needle aspirations: Are they really different?
Diana Montezuma, Sule Canberk, Ozlem Aydın, Mehmet Polat Dermirhas, André F. Vieira, Süha Goksel, Ümit İnce, Fernando Schmitt
Diagnostic Cytopathology.2018; 46(10): 859. CrossRef - Pediatric salivary gland carcinomas: Diagnostic and therapeutic management
Céleste Rebours, Vincent Couloigner, Louise Galmiche, Odile Casiraghi, Cécile Badoual, Sabah Boudjemaa, Anthony Chauvin, Monique Elmaleh, Brice Fresneau, Sylvie Fasola, Erea‐Noël Garabédian, Thierry Van Den Abeele, Daniel Orbach
The Laryngoscope.2017; 127(1): 140. CrossRef - Agreement between rapid on‐site evaluation and the final cytological diagnosis of salivary gland specimens
S. Wangsiricharoen, S. Lekawanvijit, S. Rangdaeng
Cytopathology.2017; 28(4): 321. CrossRef - Mesenchymal neoplasms of the head and neck: a cytopathologic analysis on fine needle aspiration
James Lee, Samia Kazmi, Christopher J. VandenBussche, Syed Z. Ali
Journal of the American Society of Cytopathology.2017; 6(3): 105. CrossRef - Clinical Results of Surgical Treatment in Parotid Tumors
Ahmet Kara
Journal of Otolaryngology-ENT Research.2017;[Epub] CrossRef - Parotid gland metastases of distant primary tumours: A diagnostic challenge
Achim M. Franzen, Thomas Günzel, Anja Lieder
Auris Nasus Larynx.2016; 43(2): 187. CrossRef - Modern Radiology in the Management of Head and Neck Cancer
G.J.C. Burkill, R.M. Evans, V.V. Raman, S.E.J. Connor
Clinical Oncology.2016; 28(7): 440. CrossRef - Fine‐needle aspiration and core needle biopsy: An update on 2 common minimally invasive tissue sampling modalities
Paul A. VanderLaan
Cancer Cytopathology.2016; 124(12): 862. CrossRef - Staging and follow-up of high-grade malignant salivary gland tumours: The role of traditional versus functional imaging approaches – A review
Nicole Freling, Flavio Crippa, Roberto Maroldi
Oral Oncology.2016; 60: 157. CrossRef - Biopsy of parotid masses: Review of current techniques
Sananda Haldar, Joseph D Sinnott, Kemal M Tekeli, Samuel S Turner, David C Howlett
World Journal of Radiology.2016; 8(5): 501. CrossRef - Review on the applications of ultrasonography in dentomaxillofacial region
Şehrazat Evirgen
World Journal of Radiology.2016; 8(1): 50. CrossRef - Comprehensive Cytomorphologic Analysis of Pulmonary Adenoid Cystic Carcinoma: Comparison to Small Cell Carcinoma and Non-pulmonary Adenoid Cystic Carcinoma
Seokhwi Kim, Jinah Chu, Hojoong Kim, Joungho Han
Journal of Pathology and Translational Medicine.2015; 49(6): 511. CrossRef
- Fine-needle aspiration or core needle biopsy? A meta-analysis of diagnostic accuracy and procedural outcomes in salivary gland tumors
- Cytokeratin-Positive Gastrointestinal Stromal Tumor of Biphasic Morphology: A Case Report
- Sung Sun Kim, Yoo Duk Choi, Jae Hyuk Lee, Chan Choi
- Korean J Pathol. 2014;48(5):375-378. Published online October 27, 2014
- DOI: https://doi.org/10.4132/KoreanJPathol.2014.48.5.375
- 8,715 View
- 39 Download
- 2 Crossref
-
 PDF
PDF -
Citations
Citations to this article as recorded by- CYTOKERATINS: NOT AN EPITHELIAL ENTITY ANYMORE?
Geetpriya Kaur, Devicharan Shetty, Seema Sikka, Aparna Pathak
INTERNATIONAL JOURNAL OF SCIENTIFIC RESEARCH.2022; : 15. CrossRef - Gastrointestinal stromal tumors of the stomach in a 10-year-old child
Saeed Nasher, Fayed Al-Yousofy, Faisal Ahmed
Journal of Pediatric Surgery Case Reports.2021; 74: 102044. CrossRef
- CYTOKERATINS: NOT AN EPITHELIAL ENTITY ANYMORE?
- Cytomorphological Findings and Histological Correlation of Low-Grade Cribriform Cystadenocarcinoma of Salivary Gland in Fine-Needle Aspiration: A Case Study
- Young Sin Ko, Ja Seung Koo
- Korean J Pathol. 2013;47(6):592-595. Published online December 24, 2013
- DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.6.592
- 9,103 View
- 70 Download
- 13 Crossref
-
 Abstract
Abstract
 PDF
PDF Low-grade cribriform cystadenocarcinoma (LGCCC) of the salivary gland is a rare tumor. We report the cytologic features and histologic correlation of a patient with LGCCC. A 57-year-old man had a hardly palpable, nontender mass in the right cheek area followed over nine months. Radiologic analysis revealed a 1.2 cm multiseptated, cystic, solid nodule in an anterior superficial lobe of the right parotid gland. Fine-needle aspiration cytology revealed many irregular overlapping sheets or clusters of ductal epithelial cells forming solid, pseudopapillary, and cribriform architectures. Nuclei of the tumor cells revealed inconspicuous atypia with minimal size variation. On the basis of these findings, we confirmed a diagnosis of ductal epithelial proliferative lesion, favoring neoplasm, with uncertain malignant potential. Tumor excision was performed, revealing a tiny multicystic nodule (0.7 cm). Histopathologically, this tumor showed the characteristic morphology of LGCCC. This is the first report of cytomorphological findings of LGCCC in Korea.
-
Citations
Citations to this article as recorded by- Duct tales of a parotid gland swelling
Swati Raj, Monika Singh, Mamta Gupta, Naveen Thapliyal
Cytojournal.2023; 20: 22. CrossRef - Salivary Gland Intraductal Carcinoma: How Do 183 Reported Cases Fit Into a Developing Classification
Lester D.R. Thompson, Justin A. Bishop
Advances in Anatomic Pathology.2023; 30(2): 112. CrossRef - Intraductal carcinoma of the parotid gland
Yukiya HIRATA, Kayoko HIGUCHI, Toshitaka NAGAO, Yoko ZUKERAN, Takao KINJO, Naoki WADA
The Journal of the Japanese Society of Clinical Cytology.2022; 61(6): 431. CrossRef - Intraductal carcinomas of the salivary glands: systematic review and classification of 93 published cases
Andrea Palicelli
APMIS.2020; 128(3): 191. CrossRef - What do we know about the cytological features of pure intraductal carcinomas of the salivary glands?
Andrea Palicelli
Cytopathology.2020; 31(3): 185. CrossRef - Diagnosing Recently Defined and Uncommon Salivary Gland Lesions in Limited Cellularity Specimens: Cytomorphology and Ancillary Studies
Esther Diana Rossi, Zubair Baloch, William Faquin, Liron Pantanowitz
AJSP: Reviews and Reports.2020; 25(5): 210. CrossRef - Low-grade intraductal carcinoma of salivary glands: A systematic review of this rare entity
Francesco Giovacchini, Caterina Bensi, Stefano Belli, Maria Elena Laurenti, Martina Mandarano, Daniele Paradiso, Michele Giansanti, Antonio Tullio
Journal of Oral Biology and Craniofacial Research.2019; 9(1): 96. CrossRef - The rare entity of cystadenocarcinoma (CAC) in parotid gland: A single-center experience
Bing Guo, Yu-an Cao, Xingjun Qin, Chunyue Ma
Journal of Cranio-Maxillofacial Surgery.2019; 47(5): 826. CrossRef - Cytopathology approach to rare salivary gland lesions with oncocytic features
Siba El Hussein, Samer N. Khader
Diagnostic Cytopathology.2019; 47(10): 1090. CrossRef - Unicystic high‐grade intraductal carcinoma of the parotid gland: cytological and histological description with clinic–pathologic review of the literature
Andrea Palicelli, Paola Barbieri, Narciso Mariani, Paola Re, Stefania Galla, Raffaele Sorrentino, Francesca Locatelli, Nunzio Salfi, Guido Valente
APMIS.2018; 126(9): 771. CrossRef - Low-grade cribriform cystadenocarcinoma arising from a minor salivary gland: a case report
Masashi Kimura, Shinji Mii, Shinichi Sugimoto, Kosuke Saida, Shojiroh Morinaga, Masahiro Umemura
Journal of Oral Science.2016; 58(1): 145. CrossRef - A Case of Cystadenocarcinoma Arising from Parotid Gland
Jong Chul Hong, Tae Kyoung Koh, Min Gyoung Pak, Heon Soo Park
Korean Journal of Otorhinolaryngology-Head and Neck Surgery.2016; 59(4): 300. CrossRef - Mammary analogue secretory carcinoma of parotid gland
Atsuko NASU, Sakae HATA, Masaru FUJITA, Toyoko YAMAUCHI, Satoko NAKAMURA, Takehiro TANAKA, Kouichi ICHIMURA, Hiroyuki YANAI
The Journal of the Japanese Society of Clinical Cytology.2016; 55(2): 112. CrossRef
- Duct tales of a parotid gland swelling
- Fine-Needle Aspiration Cytology of Low-Grade Cribriform Cystadenocarcinoma with Many Psammoma Bodies of the Salivary Gland
- Ji Yun Jeong, Dongbin Ahn, Ji Young Park
- Korean J Pathol. 2013;47(5):481-485. Published online October 25, 2013
- DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.5.481
- 8,401 View
- 49 Download
- 12 Crossref
-
 Abstract
Abstract
 PDF
PDF Low-grade cribriform cystadenocarcinoma (LGCCC) is a rare salivary gland tumor that was recently defined as a variant of cystadenocarcinoma by the 2005 World Health Orgazniation (WHO) classification system. We report cytologic findings of an unusual case of LGCCC with many psammoma bodies. A 90-year-old man presented a palpable mass on his left parotid gland. Fine-needle aspiration (FNA) cytology showed tumor cells that were arranged in clusters and dispersed individually. The tumor cells showed mild atypia and had clear or dense cytoplasm with some vacuoles. Numerous psammoma bodies were noted. After surgical resection, the histologic examination revealed a mixed solid and cystic mass showing intraductal growth with focal stromal invasion. The S-100 protein expressed in the tumor cells, but smooth muscle actin and p63 were positive only in myoepithelial cells. Although LGCCCs resemble other salivary gland tumors, differentiating LGCCC during preoperative FNA is important to avoid unnecessary overtreatment.
-
Citations
Citations to this article as recorded by- Salivary Gland Intraductal Carcinoma: How Do 183 Reported Cases Fit Into a Developing Classification
Lester D.R. Thompson, Justin A. Bishop
Advances in Anatomic Pathology.2023; 30(2): 112. CrossRef - Duct tales of a parotid gland swelling
Swati Raj, Monika Singh, Mamta Gupta, Naveen Thapliyal
Cytojournal.2023; 20: 22. CrossRef - Intraductal carcinoma of the parotid gland
Yukiya HIRATA, Kayoko HIGUCHI, Toshitaka NAGAO, Yoko ZUKERAN, Takao KINJO, Naoki WADA
The Journal of the Japanese Society of Clinical Cytology.2022; 61(6): 431. CrossRef - Intraductal carcinoma of the retromolar trigone found with elevated serum CEA and CA19-9 levels: a case report
Mao KAWAKAMI, Nobuhiro UEDA, Yuka TAKAHASHI, Sho ARIKAWA, Nobuhiro YAMAKAWA, Tadaaki KIRITA
Japanese Journal of Oral and Maxillofacial Surgery.2021; 67(5): 292. CrossRef - Endoscopic trans‐pterygoid resection of a low‐grade cribriform cystadenocarcinoma of the infratemporal fossa
Vikram G. Ramjee, Landon J. Massoth, John P. Richards, Kibwei A. McKinney
World Journal of Otorhinolaryngology - Head and Neck Surgery.2020; 6(2): 115. CrossRef - Psammoma Bodies in a Large Myoepithelioma
Marcela Pessoa de Melo, Diego Filipe Bezerra Silva, Rodrigo Alves Ribeiro, Tony Santos Peixoto, Daliana Queiroga de Castro Gomes, Pollianna Muniz Alves, Cassiano Francisco Weege Nonaka, Bárbara Vanessa de Brito Monteiro
Journal of Craniofacial Surgery.2020; 31(4): e326. CrossRef - Low-grade intraductal carcinoma of salivary glands: A systematic review of this rare entity
Francesco Giovacchini, Caterina Bensi, Stefano Belli, Maria Elena Laurenti, Martina Mandarano, Daniele Paradiso, Michele Giansanti, Antonio Tullio
Journal of Oral Biology and Craniofacial Research.2019; 9(1): 96. CrossRef - What is your diagnosis? Submandibular mass in a dog
Julie Allen, Ashley M. Talley, Carol B. Grindem, Jennifer A. Neel
Veterinary Clinical Pathology.2018; 47(4): 676. CrossRef - Primary acinic cell carcinoma of the lung with psammoma bodies: A case report and review of literature
Xiu-Peng Zhang, Gui-Yang Jiang, Qing-Fu Zhang, Hong-Tao Xu, Qing-Chang Li, En-Hua Wang
Pathology - Research and Practice.2017; 213(4): 405. CrossRef - Cytology of low‐grade cribriform cystadenocarcinoma in salivary glands: Cytological and immunohistochemical distinctions from other salivary gland neoplasms
Yoshiki Ohta, Yuko Hirota, Yohko Kohno, Koji Kishimoto, Tomoko Norose, Nobuyuki Ohike, Masafumi Takimoto, Akira Shiokawa, Hidekazu Ota
Diagnostic Cytopathology.2016; 44(3): 241. CrossRef - Low-grade cribriform cystadenocarcinoma arising from a minor salivary gland: a case report
Masashi Kimura, Shinji Mii, Shinichi Sugimoto, Kosuke Saida, Shojiroh Morinaga, Masahiro Umemura
Journal of Oral Science.2016; 58(1): 145. CrossRef - A Case of Cystadenocarcinoma Arising from Parotid Gland
Jong Chul Hong, Tae Kyoung Koh, Min Gyoung Pak, Heon Soo Park
Korean Journal of Otorhinolaryngology-Head and Neck Surgery.2016; 59(4): 300. CrossRef
- Salivary Gland Intraductal Carcinoma: How Do 183 Reported Cases Fit Into a Developing Classification
- Finding and Characterizing Mammary Analogue Secretory Carcinoma of the Salivary Gland
- Min Jung Jung, Joon Seon Song, Sang Yoon Kim, Soon Yuhl Nam, Jong-Lyel Roh, Seung-Ho Choi, Sung-Bae Kim, Kyung-Ja Cho
- Korean J Pathol. 2013;47(1):36-43. Published online February 25, 2013
- DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.1.36
- 14,797 View
- 117 Download
- 73 Crossref
-
 Abstract
Abstract
 PDF
PDF Background A new tumor entity of the salivary glands, mammary analogue secretory carcinoma (MASC) with
ETV6-NTRK3 translocation, has recently been proposed. MASC was originally diagnosed as adenocarcinoma, not otherwise specified (ANOS), or acinic cell carcinoma (AciCC) by the current World Health Organization classification. We aimed to identify MASC cases by molecular tests, and to characterize their clinical, histological, and immunohistochemical features.Methods Thirty cases of MASC candidates were selected after review of 196 salivary gland tumors, and subjected to break-apart
ETV6 fluorescencein situ hybridization (FISH), and immunohistochemical study for S100 protein, gross cystic disease fluid protein 15, DOG1, estrogen receptor, and progesterone receptor.Results Valid FISH results were obtained in 23 cases, and 13 positive cases were retrieved. MASCs were histologically varied, and the most frequent features observed in 10 cases were low-grade papillary/cystic/glandular patterns, intraluminal secretory materials, ovoid/wrinkled nuclei, and relatively abundant granular eosinophilic cytoplasms, corresponding to papillary-cystic or follicular types of AciCC. All cases showed diffuse immunopositivity for S100 protein. Three cases developed recurrences, but all patients remained alive.
Conclusions MASC could be a molecularly well-defined salivary gland neoplasm, encompassing some portions of AciCC and ANOS, but its histological spectrum and clinical implication require further investigation.
-
Citations
Citations to this article as recorded by- Rare Tumors of Oral Cavity: A Case Report and Literature Review on Secretory Carcinoma of Minor Salivary Glands
Emilio Salerno, Silvio Abati, Gianluigi Arrigoni, Daniela Finocchiaro, Giorgio Gastaldi, Alessandra Lissoni, Andrea Galli, Matteo Trimarchi
Clinical and Experimental Dental Research.2025;[Epub] CrossRef - Salivary Gland Secretory Carcinoma; Review of 13 Years World‐Wide Experience and Meta‐Analysis
Eyal Yosefof, Tomer Boldes, Daniel Dan, Eyal Robenshtok, Yulia Strenov, Gideon Bachar, Thomas Shpitzer, Aviram Mizrachi
The Laryngoscope.2024; 134(4): 1716. CrossRef - Salivary gland secretory carcinoma presenting as a cervical soft tissue mass: a case report
Parisa Mokhles, Alireza Sadeghipour, Pegah Babaheidarian, Saleh Mohebbi, Zahra Keshtpour Amlashi, Mohammad Hadi Gharib, Mohammad Saeid Ahmadi, Zeinab Khastkhodaei
Journal of Medical Case Reports.2024;[Epub] CrossRef - Mammary analogue secretory carcinoma of the head and neck — Clinicopathological, imaging features and prognosis analysis
Runjia Liu, Chuanzheng Sun, Likang Zhao, Shiyu Zhou, Tao Xie, Ji Zhang, Dengpeng Tang, Lei Li, Yan Xi
Journal of Radiation Research and Applied Sciences.2024; 17(2): 100914. CrossRef - Mammary analogue secretory carcinoma involving submandibular gland: Diagnostic pitfall with review of literature
Nimisha Dhankar, Nidhi Verma, Abhinav Agarwal, Ravi Mehar, Sunil Pasricha
Journal of Cancer Research and Therapeutics.2024; 20(5): 1658. CrossRef - Metastatic salivary gland mammary analogue secretory carcinoma (MASC) of parotid gland – A rare case report in the literature review
Aynur Aliyeva, Ziya Karimov, Togay Muderris
Acta Oto-Laryngologica Case Reports.2023; 8(1): 38. CrossRef - Secretory carcinoma of minor salivary glands of buccal mucosa: A case report and review of the literature
Noshad Ali Langah, Abdul Ahad, Shayan Khalid Ghaloo, Muhammad Faisal, Raza Tasawar Hussain, Fareed Akbar Shah
International Journal of Surgery Case Reports.2023; 107: 108357. CrossRef - An Underappreciated Cytomorphological Feature of Secretory Carcinoma of Salivary Gland on Fine Needle Aspiration Biopsy: Case Report with Literature Review
Yinan Hua, Bing Leng, Kenneth E. Youens, Lina Liu
Head and Neck Pathology.2022; 16(2): 567. CrossRef - Clinicopathological investigation of secretory carcinoma cases including a successful treatment outcome using entrectinib for high-grade transformation: a case report
Kensuke Suzuki, Hiroshi Harada, Masayuki Takeda, Chisato Ohe, Yoshiko Uemura, Akihiko Kawahara, Shunsuke Sawada, Akira Kanda, Bhaswati Sengupta, Hiroshi Iwai
BMC Medical Genomics.2022;[Epub] CrossRef - DOG1 as an Immunohistochemical Marker of Acinic Cell Carcinoma: A Systematic Review and Meta-Analysis
Vincenzo Fiorentino, Patrizia Straccia, Pietro Tralongo, Teresa Musarra, Francesco Pierconti, Maurizio Martini, Guido Fadda, Esther Diana Rossi, Luigi Maria Larocca
International Journal of Molecular Sciences.2022; 23(17): 9711. CrossRef - Secretory carcinoma of the sinonasal cavity and pharynx: A retrospective analysis of four cases and literature review
Changli Yue, Xiaoli Zhao, Donglin Ma, Yingshi Piao
Annals of Diagnostic Pathology.2022; 61: 152052. CrossRef - Secretory carcinoma of the salivary gland: a multi‐institutional clinicopathologic study of 90 cases with emphasis on grading and prognostic factors
Bin Xu, Kartik Viswanathan, Kavita Umrau, Thair A. D. Al‐Ameri, Snjezana Dogan, Kelly Magliocca, Ronald A. Ghossein, Nicole A. Cipriani, Nora Katabi
Histopathology.2022; 81(5): 670. CrossRef - A systematic review of secretory carcinoma of the salivary gland: where are we?
Lísia Daltro Borges Alves, Andreia Cristina de Melo, Thayana Alves Farinha, Luiz Henrique de Lima Araujo, Leandro de Souza Thiago, Fernando Luiz Dias, Héliton Spíndola Antunes, Ana Lucia Amaral Eisenberg, Luiz Claudio Santos Thuler, Daniel Cohen Goldember
Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology.2021; 132(4): e143. CrossRef - A case report of surgical resection of secretory carcinoma in the maxillary and ethmoid sinus
Kurt Willis, Martin Bullock, Matthew H. Rigby
International Journal of Surgery Case Reports.2021; 81(C): 105750. CrossRef - High‐grade salivary gland carcinoma with the ETV6‐NTRK3 gene fusion: A case report and literature review of secretory carcinoma with high‐grade transformation
Satsuki Asai, Shinji Sumiyoshi, Yosuke Yamada, Ichiro Tateya, Toshitaka Nagao, Sachiko Minamiguchi, Hironori Haga
Pathology International.2021; 71(6): 427. CrossRef - High-grade Transformation/Dedifferentiation in Salivary Gland Carcinomas: Occurrence Across Subtypes and Clinical Significance
Alena Skalova, Ilmo Leivo, Henrik Hellquist, Abbas Agaimy, Roderick H.W. Simpson, Göran Stenman, Vincent Vander Poorten, Justin A. Bishop, Alessandro Franchi, Juan C. Hernandez-Prera, David Slouka, Stefan M. Willems, Kerry D. Olsen, Alfio Ferlito
Advances in Anatomic Pathology.2021; 28(3): 107. CrossRef - Radiological features of head and neck mammary analogue secretory carcinoma: 11 new cases with a systematic review of 29 cases reported in 28 publications
Ryo Kurokawa, Mariko Kurokawa, Akira Baba, Yoshiaki Ota, Toshio Moritani, Ashok Srinivasan
Neuroradiology.2021; 63(11): 1901. CrossRef - A case of secretory carcinoma of the salivary glands in the lower lip
Reiko OHARA, Haruki SATO, Kensuke NAGANAWA, Taihei HAYAKAWA, Tatsuya KATAOKA, Ichiro OH-IWA
Japanese Journal of Oral and Maxillofacial Surgery.2021; 67(2): 83. CrossRef - Undifferentiated and dedifferentiated head and neck carcinomas
Alessandro Franchi, Alena Skalova
Seminars in Diagnostic Pathology.2021; 38(6): 127. CrossRef - Cytopathological Findings of Secretory Carcinoma of the Salivary Gland and the Diagnostic Utility of Giemsa Staining
Yuria Egusa, Midori Filiz Nishimura, Satoko Baba, Kengo Takeuchi, Takuma Makino, Tomoyasu Tachibana, Asami Nishikori, Azusa Fujita, Hiroyuki Yanai, Yasuharu Sato
Diagnostics.2021; 11(12): 2284. CrossRef - Diagnosis and treatment of secretory carcinoma arising from the oral minor salivary gland
Masaru Ogawa, Satoshi Yokoo, Takahiro Yamaguchi, Keisuke Suzuki, Mai Seki-Soda, Takahiro Shimizu, Jun Kurihara, Takaya Makiguchi
Medicine.2021; 100(51): e28390. CrossRef - A biphasic sessile mass of the buccal mucosa
Tiffany M. Peters, James A. Phero, Brent A. Golden, Alice E. Curran
Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology.2020; 130(6): 612. CrossRef - Don't stop the champions of research now: a brief history of head and neck pathology developments
Lester D.R. Thompson, James S. Lewis, Alena Skálová, Justin A. Bishop
Human Pathology.2020; 95: 1. CrossRef - High Grade Transformation in Mucoepidermoid Carcinoma of the Minor Salivary Gland with Polyploidy of the Rearranged MAML2 Gene
Hyun Lee, Jong-Lyel Roh, Young-Jun Choi, Jene Choi, Kyung-Ja Cho
Head and Neck Pathology.2020; 14(3): 822. CrossRef - Pan‐Trk immunohistochemistry is a sensitive and specific ancillary tool for diagnosing secretory carcinoma of the salivary gland and detecting ETV6–NTRK3 fusion
Bin Xu, Mohamed R Haroon Al Rasheed, Cristina R Antonescu, Deepu Alex, Denise Frosina, Ronald Ghossein, Achim A Jungbluth, Nora Katabi
Histopathology.2020; 76(3): 375. CrossRef - Characterization of novel genetic alterations in salivary gland secretory carcinoma
Kiyong Na, Juan C. Hernandez-Prera, Jae-Yol Lim, Ha Young Woo, Sun Och Yoon
Modern Pathology.2020; 33(4): 541. CrossRef - Mammary analogue secretory carcinoma: An Indian experience of a novel entity
Zeba Nisar, JaydeepN Pol, RakhiV Jagdale, MadhuraD Phadke, GirishA Kadkol
Indian Journal of Pathology and Microbiology.2020; 63(5): 134. CrossRef - Secretory Carcinoma of Salivary Gland with High-Grade Histology Arising in Hard Palate: A Case Report
Kiyofumi Takabatake, Keisuke Nakano, Hotaka Kawai, Saori Yoshida, Haruka Omori, May Wathone Oo, Shan Qiusheng, Kenichiro Uchida, Katsuaki Mishima, Hitoshi Nagatsuka
Reports.2020; 3(2): 6. CrossRef - Secretory carcinoma of the salivary gland (mammary analogue secretory carcinoma) in children
I. V. Sidorov, I. S. Kletskaya, D. M. Konovalov
Arkhiv patologii.2020; 82(2): 43. CrossRef - Secretory carcinoma of the major salivary gland: Provincial population‐based analysis of clinical behavior and outcomes
Gareth Ayre, Martin Hyrcza, Jonn Wu, Eric Berthelet, Alena Skálová, Tom Thomson
Head & Neck.2019; 41(5): 1227. CrossRef - Higher Ki67 Index, Nodal Involvement, and Invasive Growth Were High Risk Factors for Worse Prognosis in Conventional Mammary Analogue Secretory Carcinoma
Jingjing Sun, Lizhen Wang, Zhen Tian, Yuhua Hu, Ronghui Xia, Jiang Li
Journal of Oral and Maxillofacial Surgery.2019; 77(6): 1187. CrossRef - Mammary analogue secretory carcinoma of salivary gland diagnosed on submandibular gland cytology: A case report and review of the literature
Ethar Al‐Husseinawi, Soheila Hamidpour, Evanthia Omoscharka
Cytopathology.2019; 30(3): 318. CrossRef - Secretory Carcinoma of Minor Salivary Gland in Buccal Mucosa: A Case Report and Review of the Literature
Durga Paudel, Michiko Nishimura, Bhoj Raj Adhikari, Daichi Hiraki, Aya Onishi, Tetsuro Morikawa, Puja Neopane, Sarita Giri, Koki Yoshida, Jun Sato, Masayuki Ono, Yoshitaka Kamino, Hiroki Nagayasu, Yoshihiro Abiko
Case Reports in Pathology.2019; 2019: 1. CrossRef - Estrogen Receptor, Progesterone Receptor, and HER-2 Expression in Recurrent Pleomorphic Adenoma
Ana Amélia de Souza, Albina Altemani, Ney Soares de Araujo, Lucas Novaes Texeira, Vera Cavalcanti de Araújo, Andresa Borges Soares
Clinical Pathology.2019;[Epub] CrossRef - Sinonasal Secretory Carcinoma of Salivary Gland with High Grade Transformation: A Case Report of this Under-Recognized Diagnostic Entity with Prognostic and Therapeutic Implications
Bin Xu, Ruth Aryeequaye, Lu Wang, Nora Katabi
Head and Neck Pathology.2018; 12(2): 274. CrossRef - Primary mammary analogue secretory carcinoma of the lung: a case report
Tao Huang, Jonathan B. McHugh, Gerald J. Berry, Jeffrey L. Myers
Human Pathology.2018; 74: 109. CrossRef - Salivary Secretory Carcinoma With a Novel ETV6-MET Fusion
Lisa M. Rooper, Theodoros Karantanos, Yi Ning, Justin A. Bishop, Sarah W. Gordon, Hyunseok Kang
American Journal of Surgical Pathology.2018; 42(8): 1121. CrossRef - Secretory carcinoma: The eastern Canadian experience and literature review
David Forner, Martin Bullock, Daniel Manders, Timothy Wallace, Christopher J. Chin, Liane B. Johnson, Matthew H. Rigby, Jonathan R. Trites, Mark S. Taylor, Robert D. Hart
Journal of Otolaryngology - Head & Neck Surgery.2018;[Epub] CrossRef - Newly described salivary gland tumors
Alena Skalova, Michal Michal, Roderick HW Simpson
Modern Pathology.2017; 30: S27. CrossRef - Diagnóstico, tratamiento y seguimiento de un tumor de reciente descripción: el carcinoma análogo secretor de mama (MASC) de glándula salival. A propósito de 2 nuevos casos
Marina Alexandra Gavín-Clavero, M. Victoria Simón-Sanz, Ana M. López-López, Alberto Valero-Torres, Esther Saura-Fillat
Revista Española de Cirugía Oral y Maxilofacial.2017; 39(4): 221. CrossRef - Systematic review of mammary analog secretory carcinoma of salivary glands at 7 years after description
Bacem A. Khalele
Head & Neck.2017; 39(6): 1243. CrossRef - Salivary Gland Secretory Carcinoma With High-Grade Transformation, CDKN2A/B Loss, Distant Metastasis, and Lack of Sustained Response to Crizotinib
Nicole A. Cipriani, Elizabeth A. Blair, Joshua Finkle, Jennifer L. Kraninger, Christopher M. Straus, Victoria M. Villaflor, Daniel Thomas Ginat
International Journal of Surgical Pathology.2017; 25(7): 613. CrossRef - A systematic review including an additional pediatric case report: Pediatric cases of mammary analogue secretory carcinoma
Amanda L. Ngouajio, Sarah M. Drejet, D. Ryan Phillips, Don-John Summerlin, John P. Dahl
International Journal of Pediatric Otorhinolaryngology.2017; 100: 187. CrossRef - Newly Described Entities in Salivary Gland Pathology
Alena Skálová, Douglas R. Gnepp, James S. Lewis, Jennifer L. Hunt, Justin A. Bishop, Henrik Hellquist, Alessandra Rinaldo, Vincent Vander Poorten, Alfio Ferlito
American Journal of Surgical Pathology.2017; 41(8): e33. CrossRef - Mammary Analogue Secretory Carcinoma of Salivary Glands: Diagnostic Pitfall with Distinct Immunohistochemical Profile and Molecular Features
Oliver Bissinger, Carolin Götz, Andreas Kolk, Henning A. Bier, Abbas Agaimy, Henning Frenzel, Sven Perner, Julika Ribbat-Idel, Klaus Dietrich Wolff, Wilko Weichert, Caroline Mogler
Rare Tumors.2017; 9(3): 89. CrossRef - Mammary Analogue Secretory Carcinoma of Salivary Gland. A Case Report Emphasizing its Diagnostic Histological, Immunohistochemistry and Molecular Findings
Monalisa Hui, Shantveer G Uppin, Vamshi Krishna Thamtam, Abhiram Kalle
International Journal of Head and Neck Surgery.2017; 8(4): 160. CrossRef - A case of mammary analog secretory carcinoma of the lower lip
Takako Aizawa, Taro Okui, Ken Kitagawa, Yoshikazu Kobayashi, Koji Satoh, Hideki Mizutani
Journal of Oral and Maxillofacial Surgery, Medicine, and Pathology.2016; 28(3): 277. CrossRef - Mammary analogue secretory carcinoma of parotid: Is preoperative cytological diagnosis possible?
Nikita Oza, Kintan Sanghvi, Tanuja Shet, Asawari Patil, Santosh Menon, Mukta Ramadwar, Shubhada Kane
Diagnostic Cytopathology.2016; 44(6): 519. CrossRef - Lysozyme Expression Can be Useful to Distinguish Mammary Analog Secretory Carcinoma from Acinic Cell Carcinoma of Salivary Glands
Fernanda Viviane Mariano, Camila Andrea Concha Gómez, Juliana de Souza do Nascimento, Harim Tavares dos Santos, Erika Said Egal, Victor Angelo Martins Montalli, Pablo Agustin Vargas, Oslei Paes de Almeida, Albina Altemani
Head and Neck Pathology.2016; 10(4): 429. CrossRef - Cytogenetic and immunohistochemical characterization of mammary analogue secretory carcinoma of salivary glands
Syed A. Khurram, Jemel Sultan-Khan, Neil Atkey, Paul M. Speight
Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology.2016; 122(6): 731. CrossRef - The role of DOG1 immunohistochemistry in dermatopathology
Keisuke Goto
Journal of Cutaneous Pathology.2016; 43(11): 974. CrossRef - Extended immunologic and genetic lineage of mammary analogue secretory carcinoma of salivary glands
Hao Ni, Xue-ping Zhang, Xiao-tong Wang, Qiu-yuan Xia, Jing-huan Lv, Xuan Wang, Shan-shan Shi, Rui Li, Xiao-jun Zhou, Qiu Rao
Human Pathology.2016; 58: 97. CrossRef - A New Hitherto Unreported Histopathologic Manifestation of Mammary Analogue Secretory Carcinoma: “Masked MASC” Associated With Low-grade Mucinous Adenocarcinoma and Low-grade In Situ Carcinoma Components
Fredrik Petersson, Michael Michal, Dmitry V. Kazakov, Petr Grossmann, Michal Michal
Applied Immunohistochemistry & Molecular Morphology.2016; 24(9): e80. CrossRef - Papillary-cystic pattern is characteristic in mammary analogue secretory carcinomas but is rarely observed in acinic cell carcinomas of the salivary gland
Min-Shu Hsieh, Yueh-Hung Chou, Shin-Joe Yeh, Yih-Leong Chang
Virchows Archiv.2015; 467(2): 145. CrossRef - Mammary analog secretory carcinoma, low-grade salivary duct carcinoma, and mimickers: a comparative study
Todd M Stevens, Andra O Kovalovsky, Claudia Velosa, Qiuying Shi, Qian Dai, Randall P Owen, Walter C Bell, Shi Wei, Pamela A Althof, Jennifer N Sanmann, Larissa Sweeny, William R Carroll, Gene P Siegal, Martin J Bullock, Margaret Brandwein-Gensler
Modern Pathology.2015; 28(8): 1084. CrossRef - Aspiration cytology of mammary analogue secretory carcinoma of the salivary gland
Min Jung Jung, Sang Yoon Kim, Soon Yuhl Nam, Jong‐Lyel Roh, Seung‐Ho Choi, Jeong Hyun Lee, Jung Hwan Baek, Kyung‐Ja Cho
Diagnostic Cytopathology.2015; 43(4): 287. CrossRef - A Unique Case of a Cutaneous Lesion Resembling Mammary Analog Secretory Carcinoma
Jennifer Albus, Jacqueline Batanian, Bruce M. Wenig, Claudia I. Vidal
The American Journal of Dermatopathology.2015; 37(4): e41. CrossRef - Mammary analogue secretory carcinoma of salivary glands: a new entity associated with ETV6 gene rearrangement
Hanna Majewska, Alena Skálová, Dominik Stodulski, Adéla Klimková, Petr Steiner, Czesław Stankiewicz, Wojciech Biernat
Virchows Archiv.2015; 466(3): 245. CrossRef - A comparative immunohistochemistry study of diagnostic tools in salivary gland tumors: usefulness of mammaglobin, gross cystic disease fluid protein 15, and p63 cytoplasmic staining for the diagnosis of mammary analog secretory carcinoma?
Fabrice Projetti, Magali Lacroix‐Triki, Elie Serrano, Sebastien Vergez, Béatrice Herbault Barres, Julie Meilleroux, Marie‐Bernadette Delisle, Emmanuelle Uro‐Coste
Journal of Oral Pathology & Medicine.2015; 44(4): 244. CrossRef - Mammary Analogue Secretory Carcinoma of Salivary Glands
Yohei Ito, Kenichiro Ishibashi, Ayako Masaki, Kana Fujii, Yukio Fujiyoshi, Hideo Hattori, Daisuke Kawakita, Manabu Matsumoto, Satoru Miyabe, Kazuo Shimozato, Toshitaka Nagao, Hiroshi Inagaki
American Journal of Surgical Pathology.2015; 39(5): 602. CrossRef - Cytopathological features of mammary analogue secretory carcinoma—Review of literature
Maiko Takeda, Takahiko Kasai, Kohei Morita, Mao Takeuchi, Takeshi Nishikawa, Akinori Yamashita, Shinji Mikami, Hiroshi Hosoi, Chiho Ohbayashi
Diagnostic Cytopathology.2015; 43(2): 131. CrossRef - Mammary Analog Secretory Carcinoma of Salivary Glands
Justin A. Bishop
Pathology Case Reviews.2015; 20(1): 7. CrossRef - Diagnostic utility of phosphorylated signal transducer and activator of transcription 5 immunostaining in the diagnosis of mammary analogue secretory carcinoma of the salivary gland: A comparative study of salivary gland cancers
Akihiko Kawahara, Tomoki Taira, Hideyuki Abe, Yorihiko Takase, Takashi Kurita, Eiji Sadashima, Satoshi Hattori, Ichio Imamura, Shinji Matsumoto, Hitomi Fujisaki, Kazunobu Sueyoshi, Jun Akiba, Masayoshi Kage
Cancer Cytopathology.2015; 123(10): 603. CrossRef - A Case of Mammary Analogue Secretory Carcinoma Arising from Parotid Gland
Hee Tae Kim, Cha Hee Lee, Han Su Kim, Hae Sang Park
Korean Journal of Otorhinolaryngology-Head and Neck Surgery.2015; 58(8): 563. CrossRef - WITHDRAWN: A biphasic sessile mass of the buccal mucosa
Tiffany M. Peters, Jose P. Zevallos, Brent A. Golden, Alice E. Curran
Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology.2015;[Epub] CrossRef - Mammary analogue secretory carcinoma: an evaluation of its clinicopathological and genetic characteristics
Peter P. Luk, Christina I. Selinger, Timothy J. Eviston, Trina Lum, Bing Yu, Sandra A. O’Toole, Jonathan R. Clark, Ruta Gupta
Pathology.2015; 47(7): 659. CrossRef - A case of mammary analogue secretory carcinoma arising in the submandibular region
Kotaro ISHII, Koji NAKAMATSU, Koji SATO, Chikashi MINEMURA, Wataru KUMAMARU, Hiroyo YOSHIKAWA
Japanese Journal of Oral and Maxillofacial Surgery.2015; 61(11): 564. CrossRef - DOG1, p63, and S100 protein: a novel immunohistochemical panel in the differential diagnosis of oncocytic salivary gland neoplasms in fine-needle aspiration cell blocks
Alessandra C. Schmitt, Ryan McCormick, Cynthia Cohen, Momin T. Siddiqui
Journal of the American Society of Cytopathology.2014; 3(6): 303. CrossRef - Salivary Gland Tumor “Wishes” to Add to the Next WHO Tumor Classification: Sclerosing Polycystic Adenosis, Mammary Analogue Secretory Carcinoma, Cribriform Adenocarcinoma of the Tongue and Other Sites, and Mucinous Variant of Myoepithelioma
Douglas R. Gnepp
Head and Neck Pathology.2014; 8(1): 42. CrossRef - Hepatoid differentiation in renal cell carcinoma: a rare histologic pattern with clinical significance
Jungweon Shim, Heounjeong Go, Young-Suk Lim, Kyung Chul Moon, Jae Y. Ro, Yong Mee Cho
Annals of Diagnostic Pathology.2014; 18(6): 363. CrossRef - Mammary analogue secretory carcinoma: Update on a new diagnosis of salivary gland malignancy
Roshan Sethi, Elliott Kozin, Aaron Remenschneider, Josh Meier, Paul VanderLaan, William Faquin, Daniel Deschler, Robert Frankenthaler
The Laryngoscope.2014; 124(1): 188. CrossRef - Mammary analog secretory carcinoma of salivary gland in a 5 year old: Case report
Matthew Keisling, Michael Bianchi, Judy Mae Pascasio
International Journal of Pediatric Otorhinolaryngology Extra.2014; 9(4): 163. CrossRef - Fine-Needle Aspiration Cytology of Mammary Analog Secretory Carcinoma Masquerading as Low-Grade Mucoepidermoid Carcinoma: Case Report with a Review of the Literature
Jaya Bajaj, Cecilia Gimenez, Farah Slim, Mohamed Aziz, Kasturi Das
Acta Cytologica.2014; 58(5): 501. CrossRef
- Rare Tumors of Oral Cavity: A Case Report and Literature Review on Secretory Carcinoma of Minor Salivary Glands
- Diagnostic Difficulties in Fine Needle Aspiration of Benign Salivary Glandular Lesions
- Hye Jung Jo, Hyo Jung Ahn, Soojin Jung, Hye-Kyoung Yoon
- Korean J Pathol. 2012;46(6):569-575. Published online December 26, 2012
- DOI: https://doi.org/10.4132/KoreanJPathol.2012.46.6.569
- 9,436 View
- 58 Download
- 12 Crossref
-
 Abstract
Abstract
 PDF
PDF Background The diagnostic accuracy of fine needle aspiration cytology (FNAC) of salivary lesions is relatively high, but cytologic interpretation might be confusing if the sample is lacking typical cytologic features.
Methods There were 77 cases of benign salivary lesions, consisting of pleomorphic adenoma (PA) in 61 cases, Warthin's tumor (WT) in 12 cases, and other benign lesions in 4 cases. The causes of the discrepancies between the FNAC and the histologic diagnoses were evaluated.
Results Major discrepancies were noted in 4 of the 61 PA cases, and in 1 of 12 WT cases. The causes of the major discrepancies were a mislabeled site in 1 PA and 1 WT case, and an interpretation error in 3 PA cases. Minor discrepancies were more common in the WT cases (7 of 12 cases) than in the PA cases (11 of 61 cases). The causes of the minor discrepancies were a mislabeled site in 1 PA and 1 WT case, an inadequate sample in 7 PA and 2 WT cases, a lack of typical cytomorphology in 2 PA and 2 WT cases, and an interpretation error in 1 PA and 2 WT cases.
Conclusions To increase the diagnostic accuracy in the benign salivary lesions, recognition of both characteristic and less typical cytomorphology is needed.
-
Citations
Citations to this article as recorded by- Deep Learning Based on Ultrasound Images Differentiates Parotid Gland Pleomorphic Adenomas and Warthin Tumors
Yajuan Li, Mingchi Zou, Xiaogang Zhou, Xia Long, Xue Liu, Yanfeng Yao
Ultrasonic Imaging.2025; 47(3-4): 107. CrossRef - Clinical decision making when cytology indicates a Warthin tumor
Minna Sirviö, Katri Aro, Mira Naukkarinen, Antti Mäkitie, Jussi Tarkkanen, Jetta Kelppe, Timo Atula
Scientific Reports.2024;[Epub] CrossRef - Different MRI-based radiomics models for differentiating misdiagnosed or ambiguous pleomorphic adenoma and Warthin tumor of the parotid gland: a multicenter study
Jing Yang, Qiu Bi, Yiren Jin, Yong Yang, Ji Du, Hongjiang Zhang, Kunhua Wu
Frontiers in Oncology.2024;[Epub] CrossRef - Diagnostic Accuracy of Fine Needle Aspiration Cytology (FNAC) in Salivary Gland Lesions with Histopathological Examination (HPE) Correlation in a Tertiary Care Centre in Southern India
Banumathi Kasinathan, Babu Manohar, H. Ganapathy
Indian Journal of Otolaryngology and Head & Neck Surgery.2023; 75(2): 871. CrossRef - Diagnostic performance of qualitative and radiomics approach to parotid gland tumors: which is the added benefit of texture analysis?
Federica Vernuccio, Federica Arnone, Roberto Cannella, Barbara Verro, Albert Comelli, Francesco Agnello, Alessandro Stefano, Rosalia Gargano, Vito Rodolico, Giuseppe Salvaggio, Roberto Lagalla, Massimo Midiri, Antonio Lo Casto
The British Journal of Radiology.2021;[Epub] CrossRef - Improving the diagnosis of common parotid tumors via the combination of CT image biomarkers and clinical parameters
Dan Zhang, Xiaojiao Li, Liang Lv, Jiayi Yu, Chao Yang, Hua Xiong, Ruikun Liao, Bi Zhou, Xianlong Huang, Xiaoshuang Liu, Zhuoyue Tang
BMC Medical Imaging.2020;[Epub] CrossRef Evaluation of Salivary Gland Lesions by Fine Needle Aspiration Cytology at a Tertiary Care Hospital, Western Nepal
Anuj Poudel, Bigya Shrestha, Sudeep Regmi
Pathology and Laboratory Medicine International.2020; Volume 12: 9. CrossRef- Ultrasound-guided fine-needle capillary cytology of parotid gland masses coupled with a rapid-on-site evaluation improves results
R. Barats, S. Evrard, L. Collin, S. Vergez, S. Gellée, M. Courtade-Saïdi
Morphologie.2018; 102(336): 25. CrossRef - The Value of Ultrasound-Guided Fine-Needle Aspiration Cytology by Cytopathologists in the Diagnosis of Major Salivary Gland Tumors
Shahrzad Negahban, Sadegh Shirian, Bijan Khademi, Ahmad Oryan, Roshanak Sadoughifar, Mohammadian-Panah Mohammad, Azita Aledavood, Khosrow Daneshbod, Yayha Daneshbod
Journal of Diagnostic Medical Sonography.2016; 32(2): 92. CrossRef - Salivary Gland Tumors: A Diagnostic Dilemma!
Ranjit Kumar Peravali, H. Hari Kishore Bhat, Varsha H. Upadya, Anmol Agarwal, Sushma Naag
Journal of Maxillofacial and Oral Surgery.2015; 14(S1): 438. CrossRef - Diagnostic problems of salivary gland tumors
Ruchita Tyagi, Pranab Dey
Diagnostic Cytopathology.2015; 43(6): 495. CrossRef - Pleomorphic Adenoma of the Accessory Parotid Gland: Case Report and Reappraisal of Intraoral Extracapsular Dissection for Management
Tibebu M. Tsegga, Jennifer D. Britt, Aragon R. Ellwanger
Journal of Oral and Maxillofacial Surgery.2015; 73(3): 564. CrossRef
- Deep Learning Based on Ultrasound Images Differentiates Parotid Gland Pleomorphic Adenomas and Warthin Tumors
- Osteoclast-like Giant Cell Tumor of Parotid Gland with a Carcinomatous Component: A Case Report
- Jung Wook Yang, Hyeon Cheol Kim, Jeong Hee Lee, Jong Sil Lee, Dong Chul Kim, Dae Hyun Song, Jin Pyeong Kim, Gyung Hyuck Ko
- Korean J Pathol. 2012;46(3):297-301. Published online June 22, 2012
- DOI: https://doi.org/10.4132/KoreanJPathol.2012.46.3.297
- 9,346 View
- 91 Download
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF The giant cell tumor of the salivary gland is very rare, and 20 cases have been reported in the English-language literature. We report an additional case. A 57-year old man had noticed a mass in the right parotid area for several weeks. The diagnosis using aspiration cytology was a giant cell tumor possibly with a carcinomatous component. Superficial parotidectomy was carried out. The resected parotid gland contained a 1.8 cm-sized well-circumscribed brownish tumor. Histologically the tumor consisted of evenly distributed osteoclast-like giant cells, mononuclear cells and two small foci of a carcinomatous component. The osteoclast-like giant cells and mononuclear cells were positive for vimentin and CD68, and the carcinomatous component was positive for cytokeratin and epithelial membrane antigen. There was no metastatic lesion in the cervical lymph nodes. We believe this is the first case in Korea of an osteoclast-like giant cell tumor of the parotid gland.
-
Citations
Citations to this article as recorded by- Giant cell tumour of the parotid gland: a rare, unusual entity
Erna Ahsan, Kranthi Kumar Jandrasupalli, Prashant Durgapal, Divya Yadav
BMJ Case Reports.2025; 18(4): e261751. CrossRef - Genomic alteration in rare subtype of sarcomatoid salivary duct carcinoma
Ji-Seon Jeong, Kyung-Ja Cho, Deokhoon Kim, Yoon Se Lee, Joon Seon Song
Pathology - Research and Practice.2021; 228: 153678. CrossRef - Giant cell tumor of temporomandibular joint presenting as a parotid tumor: Challenges in the accurate subclassification of giant cell tumors in an unusual location
Rongqin Ren, Sandra Mueller, Adele O. Kraft, Celeste N. Powers
Diagnostic Cytopathology.2018; 46(4): 340. CrossRef - Osteoclast‐Like Giant Cell Tumor of the Parotid Gland: Report of a Case Diagnosed on Fine‐Needle Aspiration Cytology With Histological and Immunohistochemical Findings
Poonam Elhence, Meenakshi Rao, Amit Goyal, Amit Kumar, Pushpinder S. Khera, Shilajit Bhattacharya
Diagnostic Cytopathology.2016; 44(6): 548. CrossRef - Giant cell tumour of a temporomandibular joint presenting as a parotid mass: Case report and analysis of the 19 cases in the literature
Yun-Chen Huang, Jeng-Wen Chen, Yen-Lin Chen, Pei-Jen Lou
Journal of Cranio-Maxillofacial Surgery.2014; 42(8): 1778. CrossRef - Tumeur à cellules géantes de type ostéoclastique de la parotide
S. Rammeh, I. Hergli, M.K. M’farrej, N. Znaidi, S. Nechi, R. Zermani
Revue de Stomatologie, de Chirurgie Maxillo-faciale et de Chirurgie Orale.2014; 115(3): 185. CrossRef
- Giant cell tumour of the parotid gland: a rare, unusual entity
- Diagnostic Features of Fine Needle Aspiration Cytology of Pleomorphic Adenoma, Adenoid Cystic Carcinoma, and Mucoepidermoid Carcinoma of Salivary Gland.
- Eun Sook Nam, Won Bo Jo, Jung Ho Han, Insun Kim
- J Pathol Transl Med. 1990;1(1):60-67.
- 6,765 View
- 299 Download
-
 Abstract
Abstract
 PDF
PDF - The evaluate the diagnostic findings of salivary gland tumors, we reexamined aspiration cytology smears of 7 cases of pleomorphic adenoma, 3 cases of adenoid cystic carcinoma, and 3 cases of mucoepidermoid carcinoma, performed during April 1986 to March 1990, which were comfurmed by surgical excision and histologic diagnosis. The results obtained are summarized as follows : 1. All cases of pleomorphic adenoma showed branching cellular clusters of epithelial and myoepithelial cells. Acellular elements including myxomatous and chondroid components were observed. There were no cellular pleomorphism and nucleoli. Keratinizing squamous epithelial cells and keratin pearls were noted. 2. The smears of adenoid cystic carcinoma showed cell balls or cell cords containing a central hyaline core. Nuclear atypism and the nucleoli were frequently observed. There were no keratinizing squamous epithelial cells. 3. The smears of mucoepidermoid carcinoma showed mainly sheets or clusters of intermediate cells and some mucin-producing cells. Some nuclear pleomorphism was observed. Mucinous material and many inflammatory cells were present in the background.
- Sclerosing Polycystic Adenosis of the Parotid Gland: A Case Report.
- Byung Joo Jeong, Mi Ran Kim, Zhe Long Liang, Bon Seok Koo, Jin Man Kim
- Korean J Pathol. 2011;45:S79-S83.
- DOI: https://doi.org/10.4132/KoreanJPathol.2011.45.S1.S79
- 5,216 View
- 49 Download
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF - Sclerosing polycystic adenosis (SPA) of the salivary glands is a rare entity analogous to fibrocystic disease of the breast. Less than 50 cases of SPA have been published in the literature. We report the first Korean case of SPA of the right parotid gland. A 34-year-old man presented with a slowly growing right parotid mass. Computed tomography showed a relatively well-demarcated, heterogeneously enhancing mass with multiple small calcifications. Fine needle aspiration showed cohesive sheets of epithelial cells with granular oncocytic cytoplasm and scattered lymphocytes. The parotidectomy specimen showed a 3 cm-sized solid nodular lesion with small cysts. Microscopically, the lesion was an unencapsulated mass of sclerotic fibrous tissue with cystic ducts, multiple calcifications, and lymphoplasma cell infiltration. Sclerosing adenosis and cystic ducts with frequent apocrine-like cells were noted. Familiarity with the cytologic and histological features of SPA is very important making the correct diagnosis.
-
Citations
Citations to this article as recorded by- Clinicopathological profile of sclerosing polycystic adenoma/adenosis: A systematic review
Talita de Carvalho Kimura, Reydson Alcides de Lima‐Souza, João Figueira Scarini, Luccas Lavareze, Erika Said Abu Egal, Albina Altemani, Fernanda Viviane Mariano
Head & Neck.2023; 45(9): 2449. CrossRef - Juvenile sclerosing polycystic adenosis cytologically mimicking Warthin tumor
Masataka Kawai, Tomohiro Inoue, Takaaki Yonaga, Kunio Mochizuki, Tadao Nakazawa, Keisuke Masuyama, Tetsuo Kondo
Diagnostic Cytopathology.2019; 47(11): 1208. CrossRef - A Case of Sclerosing Polycystic Adenosis of Parotid Gland
Young-Jun Kim, Jang-Won Choi, Young-Joong Kim, Soo-Kweon Koo
Korean Journal of Otorhinolaryngology-Head and Neck Surgery.2014; 57(8): 559. CrossRef
- Clinicopathological profile of sclerosing polycystic adenoma/adenosis: A systematic review
- Acinic Cell Carcinoma of the Palatine Tonsil: A Brief Case Report.
- Hun Soo Kim, Keum Ha Choi
- Korean J Pathol. 2010;44(4):441-443.
- DOI: https://doi.org/10.4132/KoreanJPathol.2010.44.4.441
- 4,284 View
- 30 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF - Acinic cell carcinoma (ACC) is a rare, low-grade malignancy of the salivary glands. Most cases occur in the major salivary glands, especially the parotid gland, with only a few cases involving the minor salivary gland previously described. A 67-year-old male patient was admitted complaining of an obstructive feeling in the throat. On examination, a lobulated mass in the tonsillar surface was noticed. Tonsillectomy was performed under general anesthesia. Histopathological examination of the mass revealed sheets of large, polygonal acinar cells with granular, slightly basophilic cytoplasm, which led to the diagnosis of ACC. Here, we present a case of low-grade ACC of the palatine tonsil, which we believe to be the first reported case of ACC in this location.
-
Citations
Citations to this article as recorded by- A case of unusual heteratopic salivary gland tissue mimicking tonsillar neoplasm and review of literature
Aysegul Sule Altindal, Nermin Unal
Journal of Otolaryngology-ENT Research.2018;[Epub] CrossRef
- A case of unusual heteratopic salivary gland tissue mimicking tonsillar neoplasm and review of literature
- Oncocytoma and Oncocytic Carcinoma of the Salivary Glands, Single Institute Experience.
- Jeong Hyeon Jo, Seung Ho Choi, Jong Lyel Roh, Soon Yuhl Nam, Sang Yoon Kim, Kyung Ja Cho
- Korean J Pathol. 2010;44(4):370-375.
- DOI: https://doi.org/10.4132/KoreanJPathol.2010.44.4.370
- 4,491 View
- 65 Download
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Oncocytic neoplasms of the salivary glands are rare and the differential diagnosis between oncocytic carcinomas (OCs) and oncocytomas is difficult. We present 5 cases of oncocytoma and 3 cases of OC of the salivary glands with clinicopathological and immunohistochemical comparisons.
METHODS
Eight cases of oncocytic neoplasms diagnosed at Asan Medical Center between 1998 and 2009 were reviewed for clinical data and histological features. Immunohistochemical staining for epidermal growth factor receptor (EGFR), human epidermal growth factor receptor 2 (Her-2), c-kit, p53, and Ki-67 was done.
RESULTS
Cytological differences between oncocytomas and OCs were not obvious, but unequivocal infiltrative growths were identified in 3 cases, rendering the diagnosis of oncocytic carcinoma. When the remaining cases were classified as oncocytomas, there was no difference in age, size, and clinical symptoms between oncocytomas and OCs. Two of 3 OCs showed strong membranous expression of c-kit, but all oncocytomas were negative. The proportion of p53-positive cells was larger in OCs than oncocytomas. Her-2 or EGFR expression was absent, and Ki-67 labeling indices were less than 1% in all cases.
CONCLUSIONS
An infiltrative growth pattern, strong membranous expression of c-kit, and an increased proportion of p53-positive cells are features that can differentiate OCs from oncocytomas of the salivary glands. -
Citations
Citations to this article as recorded by- Clinicopathological Study of Oncocytomas of Head and Neck Region: A Systematic Review
João Paulo Gonçalves de Paiva, Laura Borges Kirschnick, Daniela Giraldo Roldán, Manoela Domingues Martins, Alan Roger Santos‐Silva, Ciro Dantas Soares, Jacks Jorge
Journal of Oral Pathology & Medicine.2025; 54(8): 635. CrossRef - Primary oncocytic carcinoma of ectopic salivary gland: a unique case
E. Touli, A. Manganaris, C. Nikolaidou, I. Karasmanis
International Journal of Oral and Maxillofacial Surgery.2022; 51(4): 463. CrossRef
- Clinicopathological Study of Oncocytomas of Head and Neck Region: A Systematic Review
- Cytology of Plasmacytoid Type Myoepithelioma: Report of Two Cases.
- Na Rae Kim, Hyun Yee Cho, Seung Yeon Ha
- Korean J Pathol. 2009;43(5):489-493.
- DOI: https://doi.org/10.4132/KoreanJPathol.2009.43.5.489
- 4,645 View
- 71 Download
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF - Myoepithelioma is a rare benign tumor of salivary gland myoepithelial cells, most commonly as a spindle subtype. Here, we present two cases of fine needle aspiration cytology of plasmacytoid myoepithelioma arising from a parotid gland and a hard palate. Aspirates showed plasmacytoid cells with pink-staining, homogeneous, abundant eosinophilic cytoplasm eccentrically displacing the nucleus in cohesive and dissociated forms. Rarely, nuclear grooves and intranuclear cytoplasmic inclusions were evident. These unfamiliar cytologic findings of uncommon myoepithelioma often cause diagnostic difficulties in preoperative aspiration cytology. Recognition of those rare findings provides a reliable diagnostic clue.
-
Citations
Citations to this article as recorded by- Plasmacytoid myoepithelioma: Diagnostic algorithm and a tailored therapeutic protocol for a geriatric individual
Pratik N. Patel, Aatish Thennavan, Venkadasalapathy Narayanaswamy, Raghu Radhakrishnan
Journal of Oral and Maxillofacial Surgery, Medicine, and Pathology.2015; 27(5): 737. CrossRef - Imprint Cytology of Soft Tissue Myoepithelioma: A Case Study
Seok Ju Park, Ae Ri Kim, Mi Jin Gu, Joon Hyuk Choi, Duk Seop Shin
Korean Journal of Pathology.2013; 47(3): 299. CrossRef - Fine Needle Aspiration Cytology of Benign Salivary Gland Tumors with Myoepithelial Cell Participation: An Institutional Experience of 575 Cases
Soomin Ahn, Yuil Kim, Young Lyun Oh
Acta Cytologica.2013; 57(6): 567. CrossRef - Plasmacytoid Myoepithelioma of the Palate: Case Report
Matina T. Zormpa, Asimina S. Sarigelou, Anna N. Eleftheriou, Anthoula S. Assimaki, Alexandros E. Kolokotronis
Head and Neck Pathology.2011; 5(2): 154. CrossRef
- Plasmacytoid myoepithelioma: Diagnostic algorithm and a tailored therapeutic protocol for a geriatric individual
- Fine Needle Aspiration Cytology of the Salivary Gland: An analysis of 221 cases .
- Ayoung Park, Hee Kyoung Kim, Dong Won Kim, So Young Jin, Dong Wha Lee
- J Pathol Transl Med. 1999;10(2):133-143.
- 1,983 View
- 16 Download
-
 Abstract
Abstract
 PDF
PDF - Fine needle aspiration cytology of the salivary lesions was performed on 221 patients at Soonchunhyang University Hospital for 10 years. Of 221 aspirates, 6 aspirates(2.7%) were inadequate, 116 cases(52.5%) were non-neoplastic lesions, 76(34.4%) cases were benign neoplasms and 23 cases(10.4%) were malignant neoplasms. The cytologic diagnoses could be correlated with histologic findings in 58 cases. FNAC correctly discriminated between neoplastic and nonneoplastic lesions in fifty-seven lesions and failed in a case, and overall accuracy, sensitivity, and specificity were 98.3%, 98.0%, and 100.0%. FNAC correctly discriminated malignant neoplasms from benign neoplastic/non- neoplastic lesions in fifty-three cases and failed in five cases, and overall accuracy, sensitivity, and specificity were 91.3%, 72.7%, and 95.7%. Among three false negative cases, two mucoepidermoid carcinomas were misdiagnosed as mucocele and benign neoplasm, and an acinic cell carcinoma were misdiagnosed as Warthin's tumor. Two false positive cases were a Warthin's tumor misdiagnosed as squamous cell carcinoma and a pleomorphic adenoma misinterpretated as suggestive of malignancy. In conclusion, diagnostic accuracy of FNAC of salivary lesions is high, and the possibilities of low grade mucoepidermoid carcinoma and acinic cell carcinoma should be considered on hypocellular smears with mucoid or fluidy background.
- Fine Needle Aspiration Cytology of Small Cell Carcinoma of the Parotid Gland: A Case Report .
- Chan Kwon Jung, Eun Sun Jung, Youn Soo Lee, Sun Moo Kim, Byung Kee Kim
- J Pathol Transl Med. 1999;10(2):163-167.
- 1,976 View
- 23 Download
-
 Abstract
Abstract
 PDF
PDF - Primary small cell carcinoma of the salivary gland is a rare neoplasm that accounts for approximately 1.8% of all primary major salivary gland malignancies. Because of its rarity, it is difficult to diagnose small cell carcinoma of the parotid gland by fine needle aspiration cytology(FNAC). We experienced a case of primary small cell carcinoma of the parotid gland in a 72-year-old woman who presented with two palpable masses of the left infraauricular and ocular regions of two to three month's duration, respectively. Aspirate smears from the left infraauricular area were highly cellular on necrotic and lymphocytic background and showed individually dispersed cells or three-dimensional clusters of small cells. The tumor cells were round to oval with a very high nucleocytoplasmic ratio. Nuclei were about two times the size of lymphocytes and had uniformly dispersed but hyperchromatic to pyknotic chromatin. Nucleoli were occasionally visible but were generally inconspicuous. Numerous mitotic figures were detected. The clusters of these small tumor cells exhibited angular nuclear molding, irregular nuclear outlines, and occasionally rosette like arrangement. The tumor was confirmed by histology and immunohistochemistry.
- Fine Needle Aspiration Cytology of Papillary-Cystic Variant of Acinic Cell Carcinoma of Salivary Gland: A Case Report .
- Ah Won Lee, Jin Young Yoo, Seok Jin Kang, Byung Kee Kim
- J Pathol Transl Med. 2001;12(1):45-48.
- 1,987 View
- 26 Download
-
 Abstract
Abstract
 PDF
PDF - Acinic cell carcinoma(ACC) is the third common malignancy in major salivary gland. Fine needle aspiration cytology is a useful tool for the diagnosis of salivary gland lesions. However, some low grade malignancies, such as ACC and mucoepidermoid carcinoma show relatively high false negative rate, mainly due to deceptively benign cytomorphologic appearance. We experienced a papillary-cystic variant of ACC, having different cytopathologic features compared with those of classic ACC. Our case showed monolayered sheets and papillary clusters without any acinic structures or naked nuclei of the tumor cells. Foamy proteinaceous material was seen in the background. The tumor cells had a large amount of granular cytoplasm and eccentric nuclei. Many vacuolated or clear cells were also noted.
- Fine Needle Aspiration Cytology of Salivary Duct Carcinoma with Calcification in Submandibular Gland: A Case Report .
- Ki Jung Yun, Weon Cheol Han, Hyang Jeong Jo, Kwang Man Lee
- J Pathol Transl Med. 2001;12(1):49-52.
- 2,040 View
- 26 Download
-
 Abstract
Abstract
 PDF
PDF - Salivary duct carcinoma is an uncommon aggressive malignant epithelial neoplasm with similarity to intraductal carcinoma of the breast. This neoplasm occurs most often in the parotid gland of middle-aged and older males. About 7% of reported tumors occured in the submandibular gland. The report of salivary duct carcinoma with calcification is rare. We report a case of salivary duct carcinoma with calcification in the submandibular gland. The patient was a 73-year-old male with a mass of the right submandibular gland for 1 year. On the fine needle aspiration cytology, the aspirate showed scant cellularity, small clusters of tumor cells, and scattered small calcifications. Nuclei of the tumor cells showed mild pleomorphism and round to oval in shape, and cytoplasm was abundant and finely granular. Nucleoli were indistinct and necrosis was not noted. There were no cribriform or papillary arrangements of tumor cells. Cytologic findings of salivary duct carcinoma are variable depending on histologic findings, and calcifications could be an additional cytologic finding.
- Combined Epithelial-Myoepithelial Carcinoma and Basal Cell Adenocarcinoma of the Parotid Gland: A case report.
- Young Kyung Bae, Dong Sug Kim, Jang Soo Suh, Jae Yun Ro
- Korean J Pathol. 1999;33(6):453-456.
- 2,022 View
- 32 Download
-
 Abstract
Abstract
 PDF
PDF - Epithelial-myoepithelial carcinoma and basal cell adenocarcinoma are uncommon, low-grade malignant epithelial neoplasms of salivary gland. They occur predominantly in the parotid glands with frequent recurrences and occasional distant metastases. We report an unusual case of combined epithelial-myoepithelial carcinoma and basal cell adenocarcinoma within the same mass of the parotid gland in a 32-year-old woman. To the best of our knowledge, this is the first such a combined carcinoma case.
- Quality Assuarance on Fine Needle Aspiration Cytology of Malignant Salivary Gland Neoplasms.
- Young Hyeh Ko, Young Lyun Oh
- J Pathol Transl Med. 2004;15(1):40-44.
- 2,007 View
- 11 Download
-
 Abstract
Abstract
 PDF
PDF - To evaluate the quality of fine needle aspiration cytology diagnosis on malignant salivary gland neoplasms, cytologic findings were correlated with histologic diagnosis of 56 surgically removed malignant salivary gland tumors. Seven cases (12.5%) were insufficient, 23 cases (41.1%) were diagnosed as malignant, 17 (30.4%) cases were accurately diagnosed by histologic subtype, and 9 cases (16%) were diagnosed as benign. Five out of 9 false negative cases were misdiagnosed as pleomorphic adenomas. Except the cases with insufficient specimen, overall sensitivity was 81.6%, and the sensitivity varied according to the histologic subtype; 91% in salivary duct carcinoma, 100% in carcinoma ex pleomorphic adenoma, 50% in mucoepidermoid carcinoma, 63% in adenoid cystic carcinoma, and 50% in acinic cell carcinoma. The diagnostic accuracy differed among cytopathologists irrespective of periods after acquisition of board of pathologists. These results confirm that salivary gland neoplasm can be easily misdiagnosed in fine needle aspiration cytology and a great caution should be given in diagnosing the benign appearing salivary aspirates to avoid under-diagnosis of malignant neoplasm with low grade cytologic atypia.
- A Cytologic Study of Fine Needle Aspiration Biopsy of Salivary Gland Diseases.
- Mi Jin Kim, Tae Suk Lee
- J Pathol Transl Med. 1994;5(2):120-129.
- 1,863 View
- 14 Download
-
 Abstract
Abstract
 PDF
PDF - Fine needle aspiration biopsy cytology is a widely recognized and useful technique which can provide diagnosis in lesions of the head and neck, enabling appropriate management plans for individual patient to be made. Fifty one fine needle aspirates from salivary gland masses were examined. Four aspirates(8%) were inadequate for examination. Of the remaning 47 samples, 42 cases(82%) were benign lesions which consist of 30 pleomorphic adenoma(58%), 7 inflammatory lesion(14%) 4 Warthin's tumor (8%) and 1 benign lesion(2%). Two cases(4%) were atypical lesions. Three case(6%) were malignant lesions consisting of 2 adenoid cystic carcinomas(4%) and 1 mucoepidermoid carcinoma(2%). The cytologic diagnoses were compared with the subsequent histologic diagnosis of surgical resected specimen in 24 cases. 19 cases of 21 aspirates from benign tumors were correctly diagnosed by fine needle aspiration cytology, with a specificity of 90%. All 3 aspriates from the 3 patients with malignant tumor were correctly diagnosed by fine needle aspiration cytology, with a sensitivity at 100%. Overall acurracy was 88%. Diagnostic error was encountered in adenoid cystic carcinoma, mucoepidermoid carcinoma and warthin's tumor. Correct histologic diagnosis was made in 86% of benign tumors(84% for pleomorphic adenoma and 100% for Warthin's tumor) and in 100% of malignant tumors.
- Rosai-Dorfman Disease of the Nose and Salivary Gland: A case report.
- Mee Sook Roh, Jin Sook Jeong, Sook Hee Hong
- Korean J Pathol. 1999;33(12):1203-1206.
- 2,211 View
- 20 Download
-
 Abstract
Abstract
 PDF
PDF - Rosai-Dorfman disease (RDD) is a rare type of benign histiocytosis characterized histologically by intracellular engulfment of lymphocytes. Extranodal RDD may occur as a part of generalized process involving lymph nodes or may involve extranodal sites independent of the lymph node status. We have experienced a case of extranodal Rosai-Dorfman disease of the nose as an initial lesion prior to nodal involvement. The patient was a 20-year-old woman who complained of nasal obstruction for 4 years, remotely, and left submandibular mass for 3 months, recently. Histologically, the lesion taken from nasal cavity, submandibular gland and left upper jugular lymph node all showed an heavy infiltrate consisted of plasma cells, lymphocytes and sheets of macrophages with abundant pale cytoplasm, which replaced organ architecture. The associated focal fibrosis made it difficult to differentiate from inflammatory pseudotumor. Some macrophages demonstrated phagocytosis of lymphocytes, plasma cells and occasionally neutrophils. The macrophages were strongly positive for S-100 protein.
- Cytologic Diagnosis of Basaloid Neoplasms of Salivary Gland.
- Kyung Ja Cho
- J Pathol Transl Med. 2005;16(2):67-74.
- 2,228 View
- 37 Download
-
 Abstract
Abstract
 PDF
PDF - Although fine needle aspiration cytology (FNAC) has become one of the primary tools for diagnosing salivary gland lesions, some of these methods continue to confuse pathologists. The most common problems occur so-called basaloid neoplasms. Basal cell adenomas are frequently misdiagnosed as pleomorphic adenomas, and in worse cases, as adenoid cystic carcinomas. The cytologic diagnostic accuracy of basaloid neoplasms could be increased by a better understanding of the histology and the nature of the tumor cells. These are displayed well in aspiration smears. A consideration of differential points on the basis of the epithelial-stromal relationship is offered in this paper.
- Fine Needle Aspiration Cytology of High Grade Neoplasm and Spindle Cell Lesion of Salivary Gland.
- Young Lyun Oh
- J Pathol Transl Med. 2005;16(2):75-87.
- 1,949 View
- 21 Download
-
 Abstract
Abstract
 PDF
PDF - Fine needle aspiration cytology (FNAC) is a very useful tool in the preoperative diagnosis of lesions of the salivary gland. Surgical therapy of high-grade malignancies (salivary duct carcinoma, mucoepidermoid carcinoma, squamous cellcarcinoma, carcinoma ex pleomorphic adenoma, small cell carcinoma, and sebaceous carcinoma) is different from that of benign lesions or low-grade malignancies. Therefore, the recognition of high-grade malignancies is important in salivary gland FNAC. Although recognition of high-grade malignancies of the salivary gland by FNAC is not difficult, precise classification of these malignancies is often impossible. Additionally, because of its rarity, FNAC of spindle cells and mesenchymal lesions of the salivary glands is a tool that is not familiar to many cytopathologists. The characteristic cytomorphologic features of these lesions are reviewed here with a discussion of specific diagnostic problems.
- Epstein-Barr Virus Associated Lymphoepithelial Carcinoma of the Parotid Gland: A case report.
- Kwang Il Kim, Young Sik Kim, In Sun Kim
- Korean J Pathol. 1998;32(2):150-152.
- 2,288 View
- 21 Download
-
 Abstract
Abstract
 PDF
PDF - Lymphoepithelial carcinoma is a rare subtype of undifferentiated carcinoma in the salivary gland. The incidence of lymphoepithelial carcinoma is about 0.4% among the patients with major salivary gland tumors. It has a racial preference; about 75% of the patients are of Mongolian ancestry. We report a case of lymphoepithelial carcinoma arising in the left parotid gland of a 52-year-old man. Grossly, the tumor was relatively well demarcated, gray-white, and solid. Microscopically, the irregular shaped syncytial tumor cell islands were evident within lymphoplasma cell-rich and desmoplastic stroma. The carcinoma cells had large vesicular nuclei and prominent nucleoli. The tumor invaded the surrounding salivary gland tissue. Epstein-Barr virus (EBV) was demonstrated by in situ hybridization for EBV-encoded RNA-1 (EBER-1) and polymerase chain reaction for EBV nuclear antigen-1 (EBNA-1).
- Papillary Cystadenocarcinoma In The Retromolar Area: A Brief Case Report.
- Dongchul Kim
- Korean J Pathol. 2005;39(6):433-436.
- 1,879 View
- 18 Download
-
 Abstract
Abstract
 PDF
PDF - Salivary gland papillary cystadenocarcinomas are rare lesions, and particularly when they are found in the oral cavity. They have been recognized as being low grade carcinomas of the salivary glands. The author reports here on a case of recurrent papillary cystadenocarcinoma in the right retromolar area. The initial mass had multicystic and papillary structures with low grade features. The recurred mass showed basically the same histologic features. However the layer of papillae and the solid portion were increased and the tumor cells were larger and more pleomorphic with prominent nucleoli and frequent mitoses. Focal comedo type tumor necrosis and spindle cell proliferations in the surrounding soft tissue were present. This is a very rare report of a minor salivary gland papillary cystadenocarcinoma in a Korean, and the morphologic dedifferentiation was accompanied by the clinical recurrence.
- Cytologic Findings of Epithelial-Myoepithelial Carcinoma of the Salivary Gland: A Case Report.
- Eun Sook Nam, Gu Kang, Hyung Sik Shin
- J Pathol Transl Med. 1996;7(1):64-68.
- 2,353 View
- 35 Download
-
 Abstract
Abstract
 PDF
PDF - The report of aspiration cytologic findings of epithelial-myoepithelial carcinoma(EMC) in the salivary gland is extremely rare. We present a case of fine needle aspiration cytology(FNAC) from EMC in the right submandibular gland of a 46 years old male patient. Neck CT scan revealed a confined lesion in the submandibular gland without enlargement of the regional lymph node. FNAC from the tumor showed several three-dimensional cellular clusters with admixed normal acinar cells. They frequently formed branching tubular structures composed of two type of cells; darker cells having eosinophilic scanty cytoplasm with round dense nuclei and clear cells having abudant pale cytoplasm with vesicular nuclei at the periphery of clusters. The tumor cells of both types did not show pleomorphism or mitoses. The resected submandibular gland showed an ill-defined whitish firm tumor, measuring 2 X1.5X2cm. The histology revealed an infiltrative tumor showing characteristic two cell types in a duct-like arrangement surrounded by thin basement menbrane. An inner layer of darker cells and outer layer of clear cells were postive for cytokeratin in the former and S-100 protein in the latter on the immunohistochemical stain.
- Epithelial-Myoepithelial Carcinoma of Intercalated Duct of Parotid Gland.
- Soong Deok Lee, Doo Hyun Chung, Sung Hye Park, Chul Woo Kim, Je G Chi
- Korean J Pathol. 1992;26(1):76-81.
- 2,440 View
- 20 Download
-
 Abstract
Abstract
 PDF
PDF - Epithelial-myoepithelial carcinoma of intercalated duct(origin) is a recently described tumor characterized by its typical biphasic pattern of central duct like cell and peripheral clear cell. We described a case of epithelial-myoepithelial carcinoma in a 10-year-old boy. Microscopically, the tumor showed typical biphasic pattern, diffuse proliferation of clear cells and linining epithelial cells of tubular structures. Immunohistochemically, the clear cell showed positive reaction to S-100 protein, and the epithelial cells expressed cytokeratin indicating myoepithelial and epithelial differentiation respectively. Biphasic differentiation of the tumor cells could be also proved by electronmicroscopic study.
- Fine Needle Aspiration Cytology of Acinic Cell Carcinoma of the Parotid Gland: A Case Report.
- Seok Hoon Jeon, Seung Sam Paik, Won Mi Lee, Moon Hyang Park, Jung Dal Lee
- J Pathol Transl Med. 1996;7(2):225-229.
- 2,460 View
- 71 Download
-
 Abstract
Abstract
 PDF
PDF - We experienced a case of well-differentiated acinic cell carcinoma of the parotid gland in a 65 year-old woman, which was correctly diagnosed preoperatively by fine needle aspiration(FNA) cytology. FNA cytology smears showed clusters or sheets of monomorphic acinic cells having reticulated or finely vacuolated basophilic or acidophilic cytoplasm. The cellular population was homogeneous or slightly polymorphic, having centrally located, round nuclei with finely reticular chromatin and incon- spicuous nucleoli. Herein we report this case with its histologic features and review of literatures.
- Beckwith-Wiedemann Syndrome with Unusual Sialoadenomegaly.
- Hye Seung Han, Seung Sook Lee, Suk Keun Lee, Je G Chi
- Korean J Pathol. 1996;30(10):939-942.
- 2,151 View
- 19 Download
-
 Abstract
Abstract
 PDF
PDF - Beckwith-Wiedemann syndrome is a rare clinical entity characterized by exomphalos, macroglossia, macrosomia, and renal hyperplasia/dysplasia. Although its entity is established, its etiology and obligatory features have not been settled. We report an autopsy case with the unusual involvement of the salivary gland. This infant was born to a 37-year-old mother as a normal full-term spontaneous delivery. At 11 days of age she developed with purulent eye discharge and weak sucking, and died suddenly. At autopsy the baby weighed 2,630 gm and the head circumference was 35 cm. She showed thick and prominent skin folds, bilateral aural fissures, macroglossia, hepatomegaly, cardiomegaly, dysmorphic kidneys, and nesidioblastosis. Both kidneys showed dysplastic tubules and hyperplastic cortical tissue enclosing the medulla. In this case there were characteristic findings in major and minor salivary glands with both acinar and ductal hyperplasia, and hypertrophy of mammary glands. Besides, she had generalized depletion of subcutaneous fat, immature buccal fat, patent ductus arteriosus, hyperlobation of the right lung, two accessory spleens, and hyperplasia of basophils and chromophobes in the pituitary gland. The lungs showed diffuse interstitial pneumonia and multiple fibrin thrombi. There were no adrenal cytomegaly, umbilical hernia and exophthalmos.
- Cytologic and Histologic Findings of Acinic Cell Carcinoma of the Salivary Gland Related to Malignant Behavior: 2 Cases Report .
- Sung Suk Paeng, Hee Jin Chang, Jung Il Suh, Hyo Sook Park
- J Pathol Transl Med. 1997;8(1):62-68.
- 2,074 View
- 20 Download
-
 Abstract
Abstract
 PDF
PDF - Acinic cell carcinoma is a slow-growing solid neoplasm of salivary gland. Although their cytological and histological finding is bland-looking, their biological behavior is unpredictable. We experienced two cases of acinic cell carcinoma of the salivary gland diagnosed by fine needle aspiration biopsy and confirmed by tissue examination. They showed different clinical courses. We compared their cytologic and histologic findings. The first case was a right preauricular mass in a 58 year-old female of 3 years duration. The cytologic smear revealed sheets or small clusters of monotonous cells mimicking normal serous acinar cells with little cellular pleomorphism. She underwent superficial parotid lobectomy. The tumor was a well demarcated 1.5cm sized nodular mass without infiltration into surrounding parenchyme. The second case was a left submandibular mass in a 23 year-old male of 4 years duration. The smear showed more severe pleomorphism of the tumor cells than those of previous case. Excisional biopsy was done. The excised tumor was 5.5*3.5*3cm sized multilobulated solid mass with invasion into surrounding parenchyme. The tumor recurred after 20months, thus total excision of the mass and modified radical neck dissection was carried out. From the above findings, cytologic atypism, infiltrative growth pattern and type of initial therapy may be correlated with biologic behavior.
- Pleomorphic Adenoma of the Bronchus: A case report.
- Eun Sun Park, Mi Kyung Jee, Seok Jin Gang, Byung Kee Kim, Sun Moo Kim
- Korean J Pathol. 1989;23(1):136-140.
- 1,883 View
- 19 Download
-
 Abstract
Abstract
 PDF
PDF - Pleomorphic adenoma presenting as primary lesion of the bronchus is very rarely encountered, and in our knowledge only 6 cases have been reported in the literature of the western world, and no case report has been published in Korea. Currently, we experienced a case of bronchial pleomorphic adenoma occuring in a 38 years old woman. On X-ray examination, hazy density in the right upper lobe and emphysematous change in the right lower lobe were noted. A right pneumonectomy was done under bronchoscopic diagnosis of bronchial adeoma. The pathologic examination of the present case showed a polypoid endobronchial tumor, 1.4 x 1.1 cm (with extraluminal portion, 2.2 x 1.7 cm) in the right upper bronchus. The microscopic examination revealed a pleomorphic adenoma showing same morphology as those found in the salivary gland. This case, therefore, was believed to be a genuine example of bronchial pleomorphic adenoma of salivary gland type. We compared this case with 6 cases in the literature.
- Carcinosarcoma Arising from Mixed Tumor of the Parotid Gland: A case report.
- Jae Soo Koh, Chang Won Ha, Na Hye Myoung, Kyung Ja Cho, Kyung Kyun Oh, Mi Kyung Kim, Ja June Jang
- Korean J Pathol. 1992;26(5):530-532.
- 2,353 View
- 14 Download
-
 Abstract
Abstract
 PDF
PDF - A case of true malignant mixed tumor of the parotid gland is reported. The tumor, occuring in a 55-year-old man, started to grow rapidly after a long history of parotid mass. Total parotidectomy was carried out and the resected tumor measured 5x4x3 cm with a cut surface showing grayish-white solid and myxoid appearance. Microscopically, the tumor had both carcinomatous and sarcomatous elements, the former consisting of undifferentiated carcinoma with focal areas of ductal differentiation and the latter consisting of pleomorphic sarcoma with chondrosarcomatous differentiation. A remnant of benign pleomorphic adenoma could also be identified. Immunohistochemical study demonstrated focal cytokeratin reactivity in the carcinoma cells and vimentin in sarcomatous elements. It is assumed from these clinical and histological findings that the tumor had transformed from a pre-existing benign pleomorphic adenoma.

 E-submission
E-submission
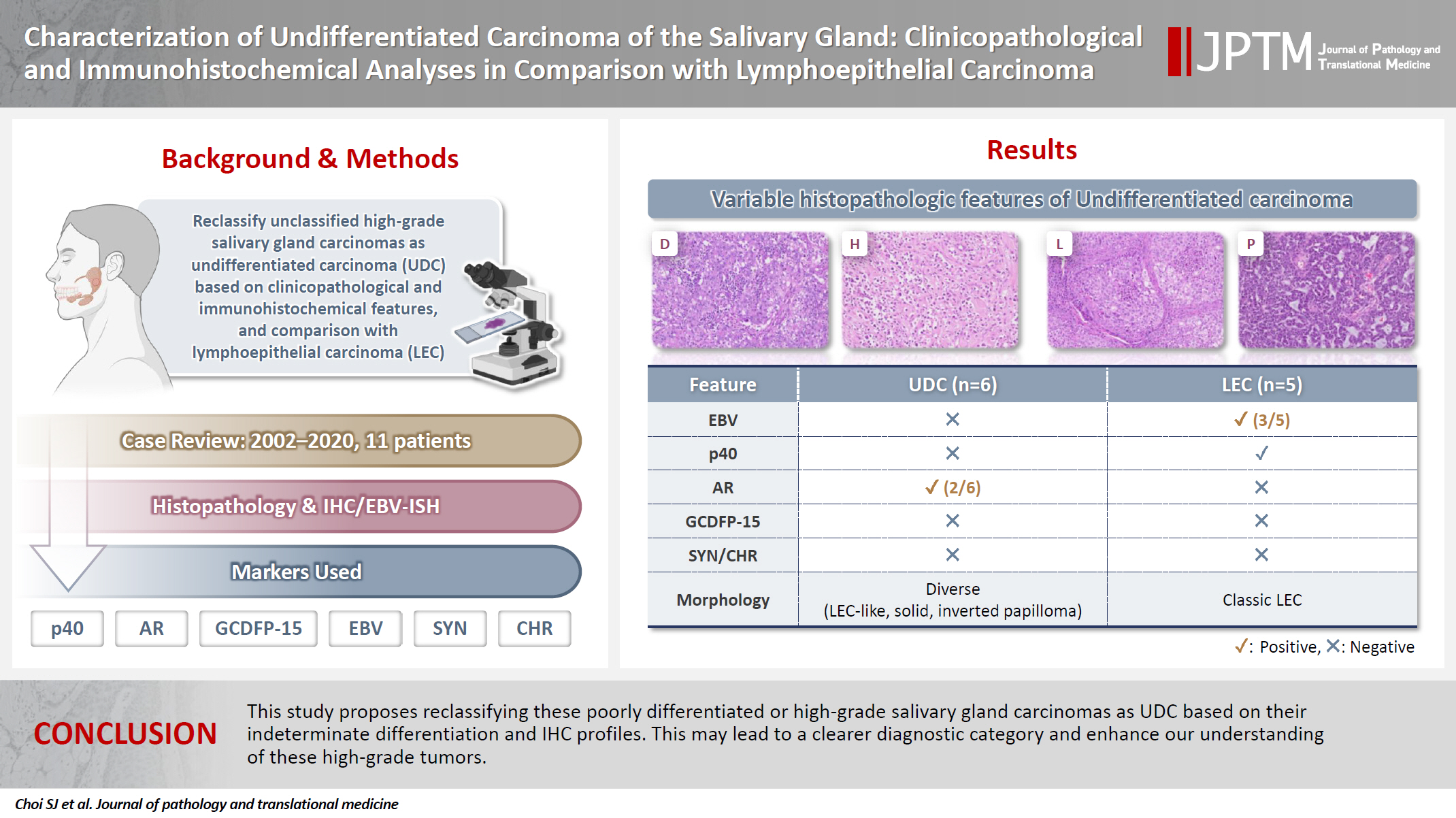





 First
First Prev
Prev



