Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 47(4); 2013 > Article
-
Original Article
SIRT1 Expression Is Associated with Good Prognosis in Colorectal Cancer - Wonkyung Jung, Kwang Dae Hong1, Woon Yong Jung, Eunjung Lee, Bong Kyung Shin, Han Kyeom Kim, Aeree Kim, Baek-hui Kim
-
Korean Journal of Pathology 2013;47(4):332-339.
DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.4.332
Published online: August 26, 2013
Department of Pathology, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea.
1Department of Surgery, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea.
- Corresponding Author: Baek-hui Kim, M.D. Department of Pathology, Korea University Guro Hospital, Korea University College of Medicine, 148 Gurodong-ro, Guro-gu, Seoul 152-703, Korea. Tel: +82-2-2626-3255, Fax: +82-2-2626-1486, maelstrom@naver.com
© 2013 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations

- Ocular surface squamous neoplasia: Update on genetics, epigenetics and opportunities for targeted therapy
Nefeli Eleni Kounatidou, Evangelos Vitkos, Sotiria Palioura
The Ocular Surface.2025; 35: 1. CrossRef - The Prognostic Impact of SIRT1, STAT3, and YAP1 in Colorectal Carcinoma
Shimaa Elkholy, Aya Abdelbary, Dina Elazab, Mohamed Elkablawy, Asmaa G. Abdou
Applied Immunohistochemistry & Molecular Morphology.2025; 33(1): 29. CrossRef - The NR3C2-SIRT1 signaling axis promotes autophagy and inhibits epithelial mesenchymal transition in colorectal cancer
Feng Li, Xing Wan, Zhigui Li, Liming Zhou
Cell Death & Disease.2025;[Epub] CrossRef - Prognostic and clinicopathological value of dbc1 expression in human cancers: a systematic review and meta-analysis
Haojia Wang, Xinhong Cheng, Bruce Xianzhuo Zhang, Yong Wang, Shuo Gao, Fanghui Ding, Xiaojing Song, Dandan Li, Haixu Ni, Yang Luo, Xun Li
Frontiers in Oncology.2025;[Epub] CrossRef - Glutamine signaling specifically activates c-Myc and Mcl-1 to facilitate cancer cell proliferation and survival
Meng Wang, Fu-Shen Guo, Dai-Sen Hou, Hui-Lu Zhang, Xiang-Tian Chen, Yan-Xin Shen, Zi-Fan Guo, Zhi-Fang Zheng, Yu-Peng Hu, Pei-Zhun Du, Chen-Ji Wang, Yan Lin, Yi-Yuan Yuan, Shi-Min Zhao, Wei Xu
Protein & Cell.2025;[Epub] CrossRef - Targeting TGF-β–Smad2/3–JNK1-mediated SIRT1 activity overcomes the chemoresistance of KRAS mutation lung cancer
Dong Hoon Shin, Minyoung Choi, Chungyong Han, Sang Soo Kim
Experimental & Molecular Medicine.2025; 57(9): 2022. CrossRef - The integrated analysis of SIRT family expression, prognostic value, and potential implications in childhood acute lymphoblastic leukemia
Xusan Xu, Zhendong Wang, Xiaoxia Wang, Wensen Zhang, Zhengqiang Luo, Xiaomei Zheng, Ronghua Pan, Ying Fu, Yajun Wang, Guochun Huang, Riling Chen, Guoda Ma
Frontiers in Oncology.2025;[Epub] CrossRef - ZMIZ1 Regulates Proliferation, Autophagy and Apoptosis of Colon Cancer Cells by Mediating Ubiquitin–Proteasome Degradation of SIRT1
Min Huang, Junfeng Wang, Zhengrong Zhang, Xueliang Zuo
Biochemical Genetics.2024; 62(4): 3245. CrossRef - Research Progress of Biological Function and Prognosis of Colorectal Cancer in Sirtuins Family
瑞阳 李
Journal of Clinical Personalized Medicine.2024; 03(04): 1805. CrossRef - Oncogenic KRAS mutation confers chemoresistance by upregulating SIRT1 in non-small cell lung cancer
Dong Hoon Shin, Jeong Yeon Jo, Minyoung Choi, Kyung-Hee Kim, Young-Ki Bae, Sang Soo Kim
Experimental & Molecular Medicine.2023; 55(10): 2220. CrossRef - Association of β-Catenin, APC, SMAD3/4, Tp53, and Cyclin D1 Genes in Colorectal Cancer: A Systematic Review and Meta-Analysis
Hongfeng Yan, Fuquan Jiang, Jianwu Yang, Ying-Kun Xu
Genetics Research.2022; 2022: 1. CrossRef - Resveratrol-related compounds: Potential for cancer and beyond
MONICA SAVIO, VALENTINA MINOIA, PAOLA FULGHIERI, LUCIA ANNA STIVALA, VIRGINIE SOTTILE
BIOCELL.2022; 46(12): 2525. CrossRef - The relationship between β-catenin and patient survival in colorectal cancer systematic review and meta-analysis
Amna Matly, Jean A. Quinn, Donald C. McMillan, James H. Park, Joanne Edwards
Critical Reviews in Oncology/Hematology.2021; 163: 103337. CrossRef - Trending topics of SIRT1 in tumorigenicity
Liz M. Garcia-Peterson, Xiaoling Li
Biochimica et Biophysica Acta (BBA) - General Subjects.2021; 1865(9): 129952. CrossRef - Surtuin 1 as a potential prognostic biomarker in very elderly patients with colorectal cancer
Guk Jin Lee, Yun Hwa Jung, Tae-Jung Kim, Yosep Chong, Seo-Won Jeong, In Kyu Lee, In Sook Woo
The Korean Journal of Internal Medicine.2021; 36(Suppl 1): S235. CrossRef - Survival and Clinicopathological Significance of SIRT1 Expression in Cancers: A Meta-Analysis
Min Sun, Mengyu Du, Wenhua Zhang, Sisi Xiong, Xingrui Gong, Peijie Lei, Jin Zha, Hongrui Zhu, Heng Li, Dong Huang, Xinsheng Gu
Frontiers in Endocrinology.2019;[Epub] CrossRef - SIRT1: a potential tumour biomarker and therapeutic target
Bin Zhao, Xin Li, Liangfu Zhou, Ye Wang, Peng Shang
Journal of Drug Targeting.2019; 27(10): 1046. CrossRef - The clinicopathological significance of SIRT1 expression in colon cancer: An immunohistochemical study and meta-analysis
Won Gi Hong, Jung-Soo Pyo
Pathology - Research and Practice.2018; 214(10): 1550. CrossRef - Sirtuin 1 and oral cancer (Review)
Shajedul Islam, Yoshihiro Abiko, Osamu Uehara, Itsuo Chiba
Oncology Letters.2018;[Epub] CrossRef - A novel SIRT1 inhibitor, 4bb induces apoptosis in HCT116 human colon carcinoma cells partially by activating p53
Ananga Ghosh, Amrita Sengupta, Guru Pavan Kumar Seerapu, Ali Nakhi, E.V. Venkat Shivaji Ramarao, Navneet Bung, Gopalakrishnan Bulusu, Manojit Pal, Devyani Haldar
Biochemical and Biophysical Research Communications.2017; 488(3): 562. CrossRef - SIRT1 gene polymorphisms and its protein level in colorectal cancer
Olfat Gamil Shaker, Miriam Safwat Wadie, Reham Maher Mohamed Ali, Ayman Yosry
Gene Reports.2017; 7: 164. CrossRef - Overexpression of SIRT1 is Associated With Poor Outcomes in Patients With Ovarian Carcinoma
David H. Mvunta, Tsutomu Miyamoto, Ryoichi Asaka, Yasushi Yamada, Hirofumi Ando, Shotaro Higuchi, Koichi Ida, Hiroyasu Kashima, Tanri Shiozawa
Applied Immunohistochemistry & Molecular Morphology.2017; 25(6): 415. CrossRef - SIRT1 suppresses colorectal cancer metastasis by transcriptional repression of miR-15b-5p
Li-Na Sun, Zheng Zhi, Liang-Yan Chen, Qun Zhou, Xiu-Ming Li, Wen-Juan Gan, Shu Chen, Meng Yang, Yao Liu, Tong Shen, Yong Xu, Jian-Ming Li
Cancer Letters.2017; 409: 104. CrossRef - TrpC5 regulates differentiation through the Ca2+/Wnt5a signalling pathway in colorectal cancer
Zhen Chen, Chunlei Tang, Yaodan Zhu, Mingxu Xie, Dongxu He, Qiongxi Pan, Peng Zhang, Dong Hua, Teng Wang, Linfang Jin, Xiaowei Qi, Yifei Zhu, Xiaoqiang Yao, Jian Jin, Xin Ma
Clinical Science.2017; 131(3): 227. CrossRef - Meta-analysis of SIRT1 expression as a prognostic marker for overall survival in gastrointestinal cancer
Shuangjie Wu, Jinghui Jiang, Jun Liu, Xinhai Wang, Yu Gan, Yifan Tang
Oncotarget.2017; 8(37): 62589. CrossRef - Prognostic and clinicopathological significance of SIRT1 expression in NSCLC: a meta-analysis
Yifei Chen, Tao Wang, Wei Wang, Jiahao Hu, Ruiting Li, Shaojun He, Jiong Yang
Oncotarget.2017; 8(37): 62537. CrossRef - The prognostic role of Sirt1 expression in solid malignancies: a meta-analysis
Changwen Wang, Wen Yang, Fang Dong, Yawen Guo, Jie Tan, Shengnan Ruan, Tao Huang
Oncotarget.2017; 8(39): 66343. CrossRef - SIRT1 induces tumor invasion by targeting epithelial mesenchymal transition-related pathway and is a prognostic marker in triple negative breast cancer
Min-Sun Jin, Chang Lim Hyun, In Ae Park, Ji Young Kim, Yul Ri Chung, Seock-Ah Im, Kyung-Hun Lee, Hyeong-Gon Moon, Han Suk Ryu
Tumor Biology.2016; 37(4): 4743. CrossRef - Survivin and SIRT1: can be two prognostic factors in chronic myeloid leukemia?
Fatemeh Salari, Javad Mohammdai-asl, Amal Saki Malehi, Ahmad Ahmadzadeh, Mohammad Ali Jalali far, Zari Tahannejad Asadi, Najmaldin Saki
Comparative Clinical Pathology.2016; 25(2): 415. CrossRef - Clinicopathological significance of SIRT1 expression in colorectal cancer: A systematic review and meta analysis
Guo Zu, Anlong Ji, Tingting Zhou, Ningwei Che
International Journal of Surgery.2016; 26: 32. CrossRef - The small molecule survivin inhibitor YM155 may be an effective treatment modality for colon cancer through increasing apoptosis
Wan Lu Li, Mi-Ra Lee, Mee-Yon Cho
Biochemical and Biophysical Research Communications.2016; 471(2): 309. CrossRef - Nuclear expression and/or reduced membranous expression of β-catenin correlate with poor prognosis in colorectal carcinoma
Shizhen Zhang, Zhen Wang, Jinlan Shan, Xiuyan Yu, Ling Li, Rui Lei, Daozhe Lin, Siqi Guan, Xiaochen Wang
Medicine.2016; 95(49): e5546. CrossRef - Association of SIRT1 and HMGA1 expression in non-small cell lung cancer
SHUANG-YAN LIN, FANG PENG
Oncology Letters.2016; 11(1): 782. CrossRef - SIRT1 is a regulator of autophagy: Implications in gastric cancer progression and treatment
Guanglin Qiu, Xuqi Li, Xiangming Che, Chao Wei, Shicai He, Jing Lu, Zongliang Jia, Ke Pang, Lin Fan
FEBS Letters.2015; 589(16): 2034. CrossRef - Stromal expression of miR-21 in T3-4a colorectal cancer is an independent predictor of early tumor relapse
Won Kyung Kang, Jin Kwon Lee, Seong Taek Oh, Sung Hak Lee, Chan Kwon Jung
BMC Gastroenterology.2015;[Epub] CrossRef - Association of SIRT1 and tumor suppressor gene TAp63 expression in head and neck squamous cell carcinoma
Keiji Kikuchi, Akira Noguchi, Rika Kasajima, Yohei Miyagi, Daisuke Hoshino, Naohiko Koshikawa, Akira Kubota, Tomoyuki Yokose, Yasuo Takano
Tumor Biology.2015; 36(10): 7865. CrossRef - Differential expressions of cancer-associated genes and their regulatory miRNAs in colorectal carcinoma
Murat Kara, Onder Yumrutas, Onder Ozcan, Ozgur Ilhan Celik, Esra Bozgeyik, Ibrahim Bozgeyik, Sener Tasdemir
Gene.2015; 567(1): 81. CrossRef - Distinctive role of SIRT1 expression on tumor invasion and metastasis in breast cancer by molecular subtype
Yul Ri Chung, Hyojin Kim, Soo Young Park, In Ae Park, Ja June Jang, Ji-Young Choe, Yoon Yang Jung, Seock-Ah Im, Hyeong-Gon Moon, Kyung-Hun Lee, Koung Jin Suh, Tae-Yong Kim, Dong-Young Noh, Wonshik Han, Han Suk Ryu
Human Pathology.2015; 46(7): 1027. CrossRef - Expression of ROR1, pAkt, and pCREB in gastric adenocarcinoma
Hyeyoon Chang, Woon Yong Jung, Youngran Kang, Hyunjoo Lee, Aeree Kim, Baek-hui Kim
Annals of Diagnostic Pathology.2015; 19(5): 330. CrossRef - miR-34a inhibits cell proliferation in prostate cancer by downregulation of SIRT1 expression
KUN DUAN, YONG-CHAO GE, XUE-PEI ZHANG, SHU-YI WU, JIN-SHUN FENG, SHI-LIN CHEN, LI ZHANG, ZHI-HAO YUAN, CHAO-HONG FU
Oncology Letters.2015; 10(5): 3223. CrossRef - Immunohistochemical Characterization of Large Intestinal Adenocarcinoma in the Rhesus Macaque (Macaca mulatta)
C. E. Harbison, F. Taheri, H. Knight, A. D. Miller
Veterinary Pathology.2015; 52(4): 732. CrossRef - Correlation and prognostic value of SIRT1 and Notch1 signaling in breast cancer
Yu-Wen Cao, Wen-Qin Li, Guo-Xing Wan, Yi-Xiao Li, Xiao-Ming Du, Yu-Cong Li, Feng Li
Journal of Experimental & Clinical Cancer Research.2014;[Epub] CrossRef - Fentanyl Increases Colorectal Carcinoma Cell Apoptosis by Inhibition of NF-κB in a Sirt1-dependent Manner
Xiu-Lai Zhang, Min-Li Chen, Sheng-Li Zhou
Asian Pacific Journal of Cancer Prevention.2014; 15(22): 10015. CrossRef - Elevated HOXB9 expression promotes differentiation and predicts a favourable outcome in colon adenocarcinoma patients
J Zhan, M Niu, P Wang, X Zhu, S Li, J Song, H He, Y Wang, L Xue, W Fang, H Zhang
British Journal of Cancer.2014; 111(5): 883. CrossRef - Prognostic Factors for Metastatic Colorectal Cancer after First-line Chemotherapy with FOLFOX-4 or FOLFIRI Regimen
Jae Hyun Kim, Pyoung Rak Choi, Seun Ja Park, Moo In Park, Won Moon, Sung Eun Kim, Gyu Won Lee
The Korean Journal of Gastroenterology.2014; 63(4): 209. CrossRef - Down-Regulation of mir-221 and mir-222 Restrain Prostate Cancer Cell Proliferation and Migration That Is Partly Mediated by Activation of SIRT1
Xiao Yang, Yingmei Yang, Rong Gan, Lingxu Zhao, Wei Li, Huaibin Zhou, Xiaojuan Wang, Jianxin Lu, Qing H. Meng, George Calin
PLoS ONE.2014; 9(6): e98833. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
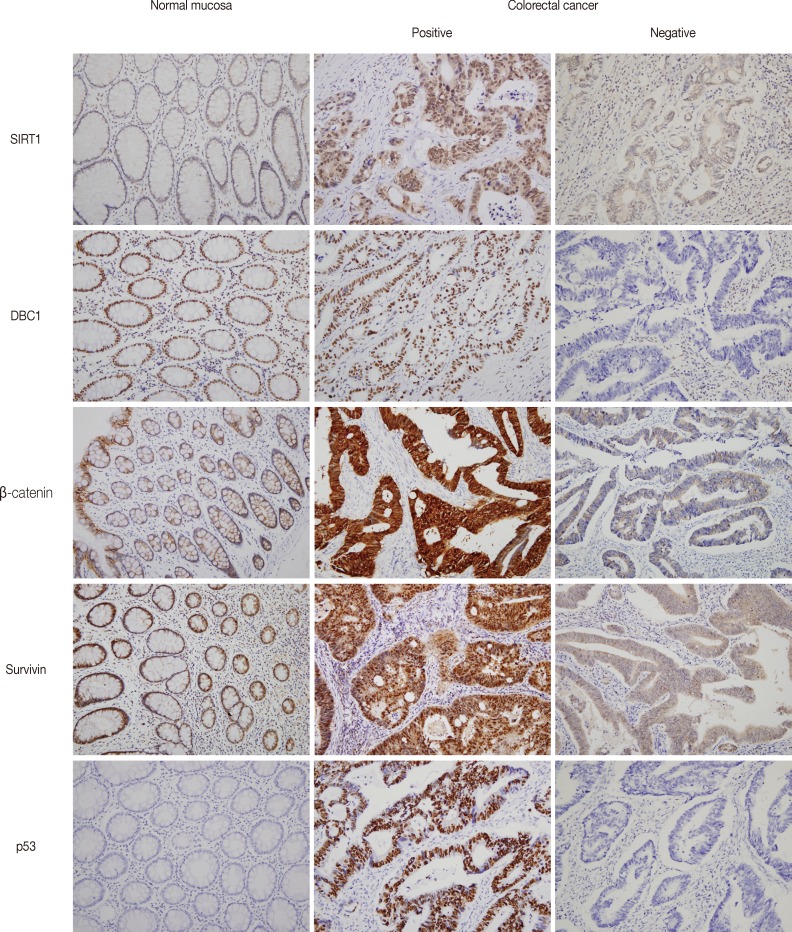
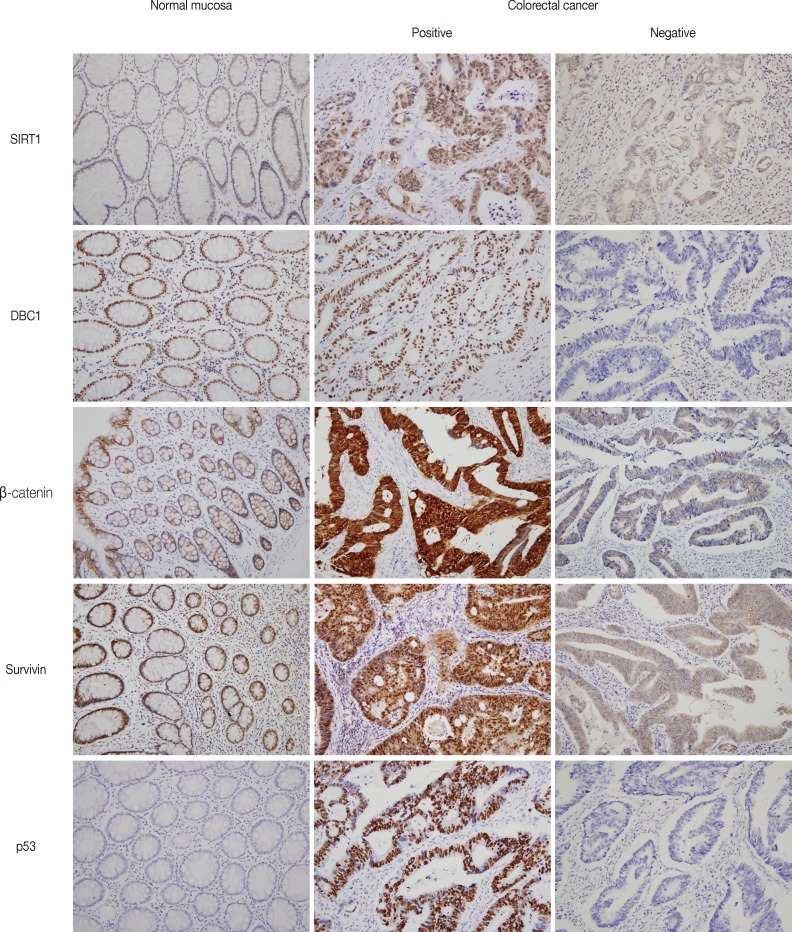
- Figure

Fig. 1
| Characteristic | No. of patients | SIRT1 |
DBC1 |
β-catenin |
Survivin |
p53 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive | p-value | Positive | p-value | Altered | p-value | Positive | p-value | Positive | p-value | ||
| Total | 349 | 235 (67) | 183 (52) | 246 (70) | 193 (55) | 190 (54) | |||||
| Age (yr) | |||||||||||
| < 65 | 186 | 129 (69) | 0.42 | 92 (49) | 0.240 | 131 (70) | 1.00 | 96 (52) | 0.161 | 109 (59) | 0.10 |
| ≥ 65 | 163 | 106 (65) | 91 (56) | 115 (71) | 97 (60) | 81 (50) | |||||
| Sex | |||||||||||
| Female | 141 | 91 (65) | 0.41 | 68 (48) | 0.230 | 91 (65) | 0.05 | 88 (62) | 0.029 | 68 (48) | 0.06 |
| Male | 208 | 144 (69) | 115 (55) | 155 (75) | 105 (50) | 122 (59) | |||||
| Location | |||||||||||
| Right | 76 | 52 (68) | 0.89 | 43 (57) | 0.438 | 41 (54) | 0.001 | 34 (45) | 0.038 | 25 (33) | < 0.001 |
| Left | 273 | 183 (67) | 140 (51) | 205 (75) | 159 (58) | 165 (60) | |||||
| TNM stage | |||||||||||
| I and II | 188 | 130 (69) | 0.49 | 98 (52) | 0.915 | 138 (73) | 0.23 | 103 (55) | 0.914 | 91 (48) | 0.018 |
| III and IV | 161 | 105 (65) | 85 (53) | 108 (67) | 90 (56) | 99 (61) | |||||
| Tumor invasion | |||||||||||
| pT1-2 | 77 | 51 (66) | 0.89 | 42 (55) | 0.700 | 53 (69) | 0.77 | 54 (70) | 0.004 | 43 (56) | 0.79 |
| pT3-4 | 272 | 184 (68) | 141 (52) | 193 (71) | 139 (51) | 147 (54) | |||||
| LN metastasis | |||||||||||
| Absence | 204 | 137 (67) | 1.00 | 106 (52) | 0.913 | 149 (73) | 0.23 | 108 (53) | 0.326 | 104 (51) | 0.12 |
| Presence | 145 | 98 (68) | 77 (53) | 97 (67) | 85 (59) | 86 (59) | |||||
| Distant metastasis | |||||||||||
| Absence | 309 | 212 (69) | 0.20 | 165 (53) | 0.401 | 224 (72) | 0.027 | 175 (57) | 0.179 | 158 (51) | 0.001 |
| Presence | 40 | 23 (58) | 18 (45) | 22 (55) | 18 (45) | 32 (80) | |||||
| Histologic grade | |||||||||||
| WD or MD | 342 | 228 (67) | 0.10 | 178 (52) | 0.452 | 244 (71) | 0.026 | 188 (55) | 0.467 | 186 (54) | 1.00 |
| PD | 7 | 7 (100) | 5 (71) | 2 (29) | 5 (71) | 4 (57) | |||||
| Characteristic | No. of patients | SIRT1 |
DBC1 |
β-catenin |
Survivin |
p53 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive | p-value | Positive | p-value | Altered | p-value | Positive | p-value | Positive | p-value | ||
| Total | 349 | 235 (67) | - | 183 (52) | - | 246 (70) | - | 193 (55) | - | 190 (54) | - |
| SIRT1 | |||||||||||
| Positive | 235 | - | - | 144 (61) | < 0.001 | 179 (76) | 0.001 | 144 (61) | 0.002 | 132 (56) | 0.36 |
| Negative | 114 | - | 39 (34) | 67 (59) | 49 (43) | 58 (51) | |||||
| DBC1 | |||||||||||
| Positive | 183 | 144 (79) | < 0.001 | - | - | 137 (75) | 0.06 | 121 (66) | < 0.001 | 97 (53) | 0.59 |
| Negative | 166 | 91 (55) | - | 109 (66) | 72 (43) | 93 (80) | |||||
| β-catenin | |||||||||||
| Altered | 246 | 179 (73) | 0.001 | 137 (56) | 0.062 | - | - | 144 (59) | 0.07 | 137 (68) | 0.48 |
| Non-altered | 103 | 56 (54) | 46 (45) | - | 49 (48) | 53 (51) | |||||
| Survivin | |||||||||||
| Positive | 193 | 144 (75) | 0.002 | 121 (63) | < 0.001 | 144 (75) | 0.07 | - | - | 98 (51) | 0.13 |
| Negative | 156 | 91 (58) | 62 (40) | 102 (65) | - | 92 (59) | |||||
| p53 | |||||||||||
| Positive | 190 | 132 (69) | 0.36 | 97 (51) | 0.592 | 137 (72) | 0.48 | 98 (52) | 0.13 | - | - |
| Negative | 159 | 103 (65) | 86 (54) | 109 (69) | 95 (60) | - | |||||
| Characteristic | No. of patients | Overall survival |
|
|---|---|---|---|
| HR (95% CI) | p-value | ||
| Age (yr) | |||
| < 65 | 186 | 1 | 0.005 |
| ≥ 65 | 163 | 2.175 (1.269-3.729) | |
| Sex | |||
| Female | 141 | 1 | 0.10 |
| Male | 208 | 1.545 (0.919-2.598) | |
| Location | |||
| Right | 76 | 1 | 0.034 |
| Left | 273 | 0.546 (0.312-0.955) | |
| TNM stage | |||
| I and II | 188 | 1 | < 0.001 |
| III and IV | 161 | 3.199 (1.812-5.647) | |
| Tumor invasion | |||
| pT1-2 | 77 | 1 | 0.024 |
| pT3-4 | 272 | 2.876 (1.149-7.203) | |
| LN metastasis | |||
| Absence | 204 | 1 | 0.001 |
| Presence | 145 | 2.392 (1.408-4.064) | |
| Distant metastasis | |||
| Absence | 309 | 1 | < 0.001 |
| Presence | 40 | 7.574 (4.423-12.967) | |
| Histologic grade | |||
| WD or MD | 342 | 1 | 0.001 |
| PD | 7 | 5.416 (1.958-14.981) | |
| SIRT1 | |||
| Negative | 114 | 1 | 0.029 |
| Positive | 235 | 0.559 (0.332-0.944) | |
| DBC1 | |||
| Negative | 166 | 1 | 0.22 |
| Positive | 183 | 1.396 (0.818-2.381) | |
| β-catenin | |||
| Non-altered | 103 | 1 | 0.008 |
| Altered | 246 | 0.493 (0.293-0.831) | |
| Survivin | |||
| Negative | 156 | 1 | 0.53 |
| Positive | 193 | 1.181 (0.696-2.005) | |
| p53 | |||
| Negative | 159 | 1 | 0.21 |
| Positive | 190 | 1.398 (0.820-2.283) | |
| Characteristic | No. of patients | Overall survival |
|
|---|---|---|---|
| HR (95% CI) | p-value | ||
| Age (yr) | |||
| < 65 | 186 | 1 | 0.001 |
| ≥ 65 | 163 | 2.563 (1.464-4.486) | |
| Location | |||
| Right | 76 | 1 | 0.15 |
| Left | 273 | 0.654 (0.362-1.180) | |
| TNM stage | |||
| I and II | 188 | 1 | < 0.001 |
| III and IV | 161 | 3.501 (1.970-6.220) | |
| Histologic grade | |||
| WD or MD | 342 | 1 | 0.002 |
| PD | 7 | 6.350 (2.021-19.952) | |
| SIRT1 | |||
| Negative | 114 | 1 | 0.031 |
| Positive | 235 | 0.540 (0.308-0.947) | |
| β-catenin | |||
| Non-altered | 103 | 1 | 0.18 |
| Altered | 246 | 0.685 (0.392-1.198) | |
Values are presented as number (%). SIRT1, silent mating type information regulation 2 homolog 1; DBC1, deleted in breast cancer 1; LN, lymph node; WD, well differentiated; MD, moderately differentiated; PD, poorly differentiated.
Values are presented as number (%). SIRT1, silent mating type information regulation 2 homolog 1; DBC1, deleted in breast cancer 1.
HR, hazard ratio; CI, confidence interval; LN, lymph node; WD, well differentiated; MD, moderately differentiated; PD, poorly differentiated; SIRT1, silent mating type information regulation 2 homolog 1; DBC1, deleted in breast cancer 1.
HR, hazard ratio; CI, confidence interval; WD, well differentiated; MD, moderately differentiated; PD, poorly differentiated; SIRT1, silent mating type information regulation 2 homolog 1.

 E-submission
E-submission