Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 47(6); 2013 > Article
-
Case Study
Peritoneal and Nodal Gliomatosis with Endometriosis, Accompanied with Ovarian Immature Teratoma: A Case Study and Literature Review - Na Rae Kim, Soyi Lim1, Juhyeon Jeong2, Hyun Yee Cho
-
Korean Journal of Pathology 2013;47(6):587-591.
DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.6.587
Published online: December 24, 2013
Department of Pathology, Gachon University Gil Medical Center, Incheon, Korea.
1Department of Gynecology and Obstetrics, Gachon University Gil Medical Center, Incheon, Korea.
2Gachon University School of Medicine, Incheon, Korea.
- Corresponding Author: Hyun Yee Cho, M.D. Department of Pathology, Gachon University Gil Medical Center, 21 Namdong-daero 774beon-gil, Namdong-gu, Incheon 405-760, Korea. Tel: +82-32-460-3865, Fax: +82-32-460-3073, hicho@gilhospital.com
© 2013 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- Gliomatosis peritonei (GP) indicates the peritoneal implantation of mature neuroglial tissue and is usually accompanied by ovarian mature or immature teratoma. Here, we report a case of ovarian immature teratoma associated with gliomatosis involving the peritoneum, lymph nodes and Douglas' pouch, where gliomatosis coexisted with endometriosis. As far as we know, only seven cases of GP have been reported as coexisting with endometriosis. Eight cases with mature glial tissue in the lymph nodes, i.e., nodal gliomatosis, have been published either in association with GP or in its absence. Metaplasia of pluripotent coelomic stem cells has been suggested to be responsible for the pathogenesis of endometriosis and GP rather than implantation metastases of ovarian teratomatous tumor with varying maturation. This theory is also applied to GP independently of ovarian teratomatous tumors. To the best of our knowledge, nodal gliomatosis coexisting with GP and also involving endometriosis has not yet been reported.
- A 34-year-old female (G2P2A0L2) visited our hospital with an eight-month history of a palpable abdominal mass. She had a history of two cesarean sections. Normal beta-human chorionic gonadotropin with increased alpha-fetoprotein (22.7 ng/mL), cancer antigen (CA) 125 (61.7 U/mL), and CA19-9 (553.7 U/mL) were observed. Contrast-enhanced computed tomography showed a large solid and cystic mass in the left lower abdomen and pelvis. A huge left ovarian tumor filling the peritoneal cavity was found during laparotomy, and yellow-colored ascites (200 mL) was expelled. The external surface of the tumor was pinkish-tan, transparent, smooth, and intact. No pelvic adhesions were observed. Multiple firm, gray-white nodules measuring 0.1 to 0.3 cm were observed on the omentum, uterine serosa and posterior cul-de-sac peritoneum. One surface hemorrhagic nodule that measured 0.3 cm was found on the right ovary. Left salpingo-oophorectomy, right wedge resection, partial omentectomy, and biopsy of the uterine serosal nodules were performed. Under the impression of ovarian malignant tumor, intraoperative frozen biopsies were taken from the omental nodules, left ovary and salpinx. For staging, pelvic lymph node sampling, partial omentectomy and posterior cul-de-sac peritoneal resection were performed.
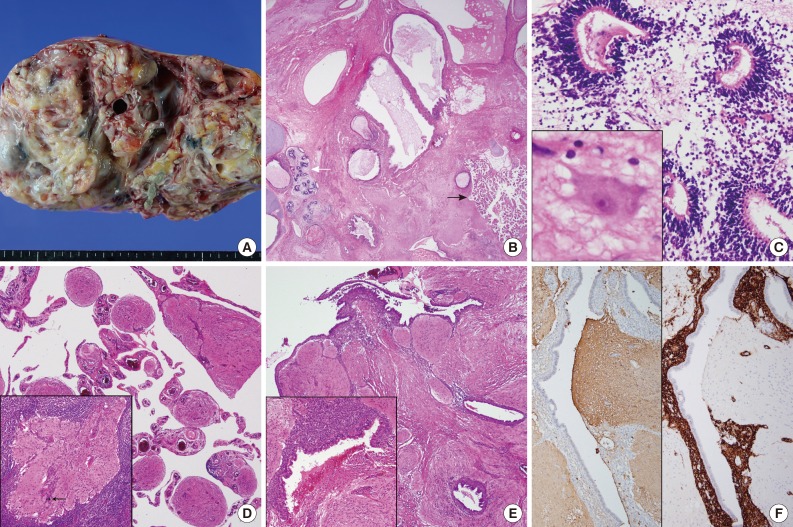
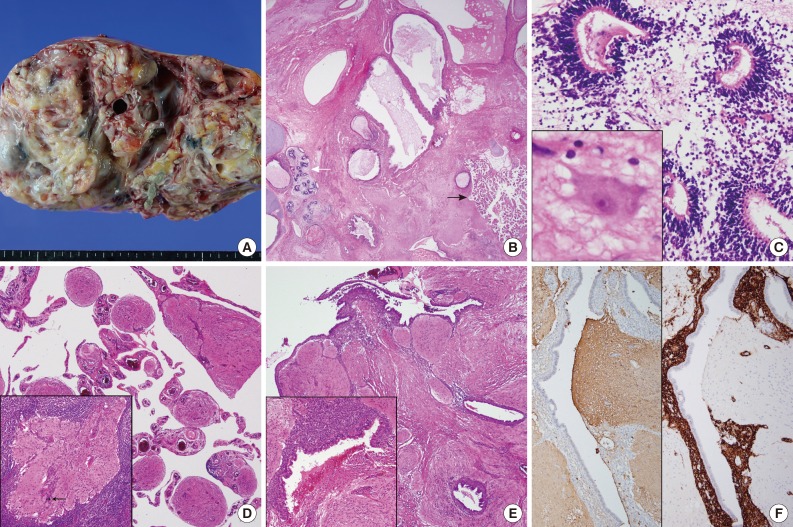
- Grossly, the excised left ovarian mass measured 21.0 cm in diameter and weighed 2,350 g. A predominantly solid mass with multicystic changes, which were filled with clear watery fluid, was observed on the cut surface (Fig. 1A). Thirty sections were taken from the ovarian tumor, and various mature tissues of tridermal lineage were demonstrated (Fig. 1B); the mass was comprised predominantly of mature glial tissue (90%) with focal immature neuroepithelial cells forming rosette-like structures with few mitosis (Fig. 1C). Some mature neurons were also observed within the glial tissue. Choroid plexuses were also found. These immature neuroectodermal foci, including primitive mesenchyma, led to the diagnosis of an immature teratoma, grade 1, according to the classification of Robboy and Scully.3 There were no other germ cell tumor components, including a yolk sac tumor. The peritoneum, cul-de-sac and uterine serosa, as well as the opposite ovarian surface, showed involvement of glial peritonei (grade 0). Nodules of the right ovary, peritoneum and omentum were composed of glial tissue (grade 0) (Fig. 1D). Out of 32 dissected lymph nodes, one left hypogastric lymph node showed one mature glial implant, i.e., nodal gliomatosis (grade 0) (Fig. 1D, inset). Endometrial glands with stromata were found within the peritoneal nodules (Fig. 1E). Some scattered psammoma bodies were observed in peritoneal and nodal gliomatosis, as well as brain tissue in the ovarian immature teratoma. Immunohistochemically, glial fibrillary acidic protein (prediluted, polyclonal, Dako, Glostrup, Denmark) (Fig. 1F, left) immunostaining confirmed the glial nature of the tissue. The endometrial stroma of endometriosis was stained with CD10 (56C6, prediluted, Dako) (Fig. 1F, right).
- Three cycles of chemotherapy of bleomycin, etoposide and cisplatin (BEP) were performed. After the surgery, alpha-fetoprotein, CA125, and CA19-9 levels reached the normal range. During the nine-month post-operative follow-up period, the patient had an uneventful course.
CASE REPORT
- GP is a rarely encountered complication of ovarian teratomas and is characterized by peritoneal implants of glial tissue usually of a low World Health Organization grade, although cases of malignant evolution or high grades have also been described.16 About 100 cases have been retrieved in the literature. To our best knowledge, nine cases of nodal gliomatosis (including the present case) were demonstrated in the pelvic or paraaortic lymph nodes;1-8 eight cases were associated with both GP and ovarian teratomatous tumors, while the remaining case was without GP.6 Another interesting phenomenon is the rare combination of GP and endometriosis, as was seen in the present case. To date, seven cases of endometriosis combined with GP have been published in the world literature.9-15 A summary of peritoneal gliomatosis coexisting with nodal gliomatosis or endometriosis is shown in Tables 1 and 2.
- The pathogenesis of GP is not yet completely understood. Lymphangeneous glial implants or direct spillage of the glial foci of an ovarian teratoma have been suggested by the previous articles.2-7 Another viewpoint proposed that GP is a metastasis of glial tissue to a focus of pre-existing endometriosis because these endometrial foci are more vascular and therefore are more receptive. However, this concept contradicts the fact that endometriosis is rare in the pediatric population, and it is unlikely because despite extensive sampling, there were only mixed endometrial and glial cells but no pure endometriosis. The pathogenesis of endometriosis and GP may be shared. Originally, the presence of GP indicated that these lesions were shared by implantation metastases of an ovarian teratomatous tumor with varying maturation.7,10-12 Metaplasia of pluripotent coelomic or Mullerian stem cells has also been suggested, but the most scientifically supported and widely accepted mechanism of endometriosis is retrograde effluent flow through the lumen of the fallopian tubes into the pelvic-peritoneal cavities during menstruation.9 Metaplasia may be induced by unknown stimuli in other uncommon peritoneal lesions, such as melanosis peritonei, etc.17 Dworak et al.11 suggested that GP temporally predisposes a patient to the development of endometriosis. A recent assay for loss of heterozygosity revealed that normal tissues and GP showed a heterozygous pattern in the microsatellite loci, whereas the associated ovarian teratomas had a homozygous pattern.18 These findings suggest that GP with or without nodal involvement is genetically unrelated to the associated teratoma but may be stimulated by these teratomas. Other rare locations such as intrathoracic gliomatosis, isolated GP independent to ovarian teratoma or one occurring after ventriculoperitoneal shunt operations also support this theory. The peritoneal mesothelium that modulates the cytoskeleton and shape is explained as subserosal multipotential subserosal cells that can replicate and differentiate diversely under unspecified stimuli.19 Mesothelial hyperplasia is commonly found when it is stimulated by pleural and peritoneal effusion.19 This mesothelial hyperplasia may be rarely seen in lymph nodes, and in these cases it would be mimicking metastatic tumors.18 Gliomatosis affecting the lymph node can be explained by this unusual location of mesothelial hyperplasia, although the present case showed no peritoneal mesothelial hyperplasia. A frequent association between GP and effusion was found; about 35 cases of GP were accompanied by ascites, and 3 cases involved pleural effusion.1,4,6,8 The frequent association with ascites or pleural effusion and co-existing nodal or GP, as shown in Table 1, may provide supporting evidence of this unusual location of gliomatosis.1,19 However, although the common association of GP with ovarian teratoma is indisputable, the reason why subperitoneal cells develop into glial islands in the presence of these ovarian tumors remains to be investigated. Another explanation of the occasionally present ascites or pleural effusion is that the pressure on the lymphatics in the tumor of the peritoneum as well as the ovary causes the fluid to escape through the superficial lymphatic vessels of the tumor or induces increased vascular permeability.19
- Clinically, intraoperatively encountered widespread, grayish tan-colored, firm, tiny nodules can be mistaken as peritoneal carcinomatosis or disseminated tuberculosis, especially in cases showing calcification or psammoma bodies like the present patient.10,11 Despite the rarity of ovarian immature teratomas with GP data and limitations, the prognosis of GP has long been regarded as good compared to that of immature teratoma without GP.14 However, the application of cisplatin-based chemotherapy regimens has dramatically improved the prognosis of immature teratomas of high grades and stages.16 Recent studies suggested that the poor prognosis in immature teratoma is correlated with incomplete resection, tumor rupture, younger age, higher stage and grade, and GP, but not with cyst spillage, enucleation, nodal gliomatosis, or microfoci of yolk sac tumor, i.e., Heifetz lesions.16
- To the best of our knowledge, peritoneal and nodal gliomatosis as well as combined endometriosis are rare phenomena that have not yet been described in the English literature.
DISCUSSION
- 1. Benirschke K, Easterday C, Abramson D. Malignant solid teratoma of the ovary: report of three cases. Obstet Gynecol 1960; 15: 512-521. PubMed
- 2. Kourie M, Roujeau J. Métastases neuroïdes matures d’un tératome ovarian. Arch Anat Pathol 1966; 14: 22-23.
- 3. Robboy SJ, Scully RE. Ovarian teratoma with glial implants on the peritoneum: an analysis of 12 cases. Hum Pathol 1970; 1: 643-653. PubMed
- 4. Nagashima K, Yamaguchi K, Hasumi K, Oota K. Malignant gliomatosis peritonei originating from cystic ovarian teratoma. Acta Pathol Jpn 1974; 24: 529-539. ArticlePubMed
- 5. Perrone T, Steiner M, Dehner LP. Nodal gliomatosis and alpha-fetoprotein production: two unusual facets of grade I ovarian teratoma. Arch Pathol Lab Med 1986; 110: 975-977. PubMed
- 6. El Shafie M, Furay RW, Chablani LV. Ovarian teratoma with peritoneal and lymph node metastases of mature glial tissue: a benign condition. J Surg Oncol 1984; 27: 18-22. ArticlePubMed
- 7. Harms D, Jänig U, Göbel U. Gliomatosis peritonei in childhood and adolescence: clinicopathological study of 13 cases including immunohistochemical findings. Pathol Res Pract 1989; 184: 422-430. PubMed
- 8. Khan J, McClennan BL, Qureshi S, Martell M, Iyer A, Bokhari SJ. Meigs syndrome and gliomatosis peritonei: a case report and review of literature. Gynecol Oncol 2005; 98: 313-317. ArticlePubMed
- 9. Albukerk JN, Berlin M, Palladino VC, Silverman J. Endometriosis in peritoneal gliomatosis. Arch Pathol Lab Med 1979; 103: 98-99.
- 10. Bässler R, Theele C, Labach H. Nodular and tumorlike gliomatosis peritonei with endometriosis caused by a mature ovarian teratoma. Pathol Res Pract 1982; 175: 392-403. ArticlePubMed
- 11. Dworák O, Knöpfle G, Varchmin-Schultheiss K, Meyer G. Gliomatosis peritonei with endometriosis externa. Gynecol Oncol 1988; 29: 263-266. ArticlePubMed
- 12. Calder CJ, Light AM, Rollason TP. Immature ovarian teratoma with mature peritoneal metastatic deposits showing glial, epithelial, and endometrioid differentiation: a case report and review of the literature. Int J Gynecol Pathol 1994; 13: 279-282. ArticlePubMed
- 13. Killeen VB, Reich H, McGlynn F, Virgilio LA, Krawitz MA, Sekel L. Pelvic gliomatosis within foci of endometriosis. JSLS 1997; 1: 267-268. PubMedPMC
- 14. Müller AM, Söndgen D, Strunz R, Müller KM. Gliomatosis peritonei: a report of two cases and review of the literature. Eur J Obstet Gynecol Reprod Biol 2002; 100: 213-222. ArticlePubMed
- 15. Alexander M, Cope N, Renninson J, Hong A, Simpson RH, Hirschowitz L. Relationship between endometriosis, endometrioid adenocarcinoma, gliomatosis peritonei, and carcinoid tumor in a patient with recurrent ovarian teratoma. Int J Gynecol Pathol 2011; 30: 151-157. ArticlePubMed
- 16. Mann JR, Gray ES, Thornton C, et al. Mature and immature extracranial teratomas in children: the UK Children's Cancer Study Group Experience. J Clin Oncol 2008; 26: 3590-3597. ArticlePubMed
- 17. Clement PB, Young RH, Oliva E, Sumner HW, Scully RE. Hyperplastic mesothelial cells within abdominal lymph nodes: mimic of metastatic ovarian carcinoma and serous borderline tumor: a report of two cases associated with ovarian neoplasms. Mod Pathol 1996; 9: 879-886. PubMed
- 18. Vortmeyer AO, Devouassoux-Shisheboran M, Li G, Mohr V, Tavassoli F, Zhuang Z. Microdissection-based analysis of mature ovarian teratoma. Am J Pathol 1999; 154: 987-991. ArticlePubMedPMC
- 19. Yáñez-Mó M, Lara-Pezzi E, Selgas R, et al. Peritoneal dialysis and epithelial-to-mesenchymal transition of mesothelial cells. N Engl J Med 2003; 348: 403-413. PubMed
REFERENCES
| No. | Reference | Age (yr)/Sex | Involved site by endometriosis | Associated ovarian or peritoneal lesion |
Increased serum oncoprotein |
Treatment | Outcome | |||
|---|---|---|---|---|---|---|---|---|---|---|
| AFP | CA125 | β-HCG | CA19-9 | |||||||
| 1 | Albukerk et al. (1979) [9] | 20/F | Cul-de-sac, uterus, pelvis, omentum | Immature teratoma (G2, 10 yr before), GP | ND | ND | ND | ND | ND | ND |
| 2 | Bassler et al. (1982) [10] | 22/F | Intestinal serosa, urinary bladder, Douglas pouch, omentum | Mat니re teratoma (12 yr before), GP | ND | ND | ND | ND | No further treatment | Recurrence of GP (1 yr) |
| 3 | Dworak et al. (1988) [11] | 9/F | Omentum, sacrouterine lesion | Immature teratoma (G1, 9 yr before), GP | WNL | WNL | WNL | ND | No further treatment | ND |
| 4 | Calder et al. (1994) [12] | 31/F | Douglas pouch, uterosacral ligament | Immature teratoma (G1, 20 yr before), GP | ND | ND | ND | ND | No further treatment | NR (4 yr) |
| 5 | Killeen et al. (1997) [13] | 30/F | Pelvic peritoneum, rectum, rectovaginal septum, uterus | Mature teratoma (G0, 16 yr before), GP | ND | ND | ND | ND | No further treatment | NR (5 yr) |
| 6 | Muller et al. (2002) [14] | 25/F | Peritoneum, greater omentum | Immature teratoma (G1, 4 yr before), GP | ND | ND | ND | ND | No further treatment | NR (5.5 yr) |
| 7 | Alexander et al. (2011) [15] | 59/F | Bilateral ovaries | Mature teratoma (23 yr before), GP, ovarian endometrioid adenocarcinoma and carcinoid, uterine endometrial mucinous adenocarcinoma | ND | ND | ND | ND | No further treatment for GP and endometriosis | ND |
| 8 | Kim et al. (present case) | 34/F | Cul-de-sac, omentum | Synchronous immature teratoma (G1), gliomatosis in the surface of the opposite ovary and uterine serosa, GP | ↑ | WNL | WNL | ↑ | No further treatment | NR (9 mo) |
| No | Reference | Age/Sex | Involved lymph node (grade of implant) | Plural or peritoneal effusion | Associated ovarian lesions or gliomatosis other than lymph node |
Serum oncoprotein |
Treatment | Outcome | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AFP | CA125 | β-HCG | CA19-9 | ||||||||
| 1 | Benirschke et al. (1960) [1] | 18yr/F | Retroperitoneal, iliac, cervical, axillary (GO) | Ascites | Ovarian mature teratoma, GP | NA | NA | NA | NA | Chemoradiotherapy | Dead (8 mo) |
| 2 | Kourie and Roujeau (1966) [2] | 9yr/F | Mesenteric (G1) | Absent | Ovarian immature teratoma (G1), GP | ND | ND | ND | ND | No further treatment | NR (5 yr) |
| 3 | Robboy and Scully (1970) [3] | 9yr/F | Mesenteric (G1) | Absent | Ovarian immature teratoma (G1), omentum, GP | ND | ND | ND | ND | No further treatment | NR (5 yr) |
| 4 | Nagashima et al. (1974) [4] | 22 yr/F | Inguinal, mesenteric, mediastinal, cervical (malignanta) | Ascites, pleural effusion | Ovarian immature teratoma (8 mo before)a, pleural gliomatosisa, GPa | NA | NA | NA | NA | Chemotherapy | Dead (8 mo) |
| 5 | Perrone et al. (1986) [5] | 10mo/F | Para-aortic (GO) | Absent | Ovarian immature teratoma (G1), no GP | ↑ | WNL | WNL | WNL | No further treatment | NR (9 mo) |
| 6 | El Shafie et al. (1984) [6] | 12 yr/F | Omental (GO) | Ascites (1 L) | Ovarian teratoma, serosa of small and large intestine, appendiceal surface, GP | WNL | WNL | WNL | WNL | No further treatment | NR (5 yr) |
| 7 | Harms et al. (1989) [7] | 13 yr/F | Para-aortic (G3) | Absent | Ovarian immature teratoma (G1), GP, hepatic serosa | ND | ND | ND | ND | Chemotherapy | NR |
| 8 | KUan et al. (2005) [8] | 23 yr/F | Lymph node (GO) | Pleural effusion, ascites | Ovarian immature teratoma (G1), omentum, GP | ↑ | ↑ | ND | ↑ | Chemotherapy | ND |
| 9 | Kim et al. (present case) | 34 yr/F | Hypogastric (GO) | Ascites (200 mL) | Ovarian immature teratoma (G1), GP | ↑ | WNL | WNL | ↑ | No further treatment | NR (9 mo) |
AFP, alpha-fetoprotein; CA125, cancer antigen 125; β-HCG, beta-human chorionic gonadotropin; CA19-9, cancer antigen 19-9; F, female; G0, grade 0; GP, gliomatosis peritonei; NA, unavailable; G1, grade 1; ND, not described; NR, no recurrence; WNL, within normal limits; G3, grade 3.
aGrading is not described in the article.
Figure & Data
References
Citations

- Mimics of primary ovarian cancer and primary peritoneal carcinomatosis – A pictorial review
B. Lawson, I. Rajendran, J. Smith, A. Shakur, V. Sadler, T.J. Sadler, H.C. Addley, S. Freeman
Clinical Radiology.2024; 79(10): 736. CrossRef - Ovarian Immature Teratoma With Nodal Gliomatosis: A Case Report and Literature Review
Marwa Alna’irat, W. Glenn McCluggage, Maysa Al-Hussaini
International Journal of Gynecological Pathology.2023; 42(6): 627. CrossRef - Germ Cell Tumors of the Ovary: A Review
Preetha Ramalingam
Seminars in Diagnostic Pathology.2023; 40(1): 22. CrossRef - Immature Teratoma with Gliomatosis Peritonei Arising in a Young Girl: Report of a Rare Case and Review of Literature
Isheeta Ahuja, Ruchi Rathore, Neerja Bhatla, Sandeep R. Mathur
Indian Journal of Gynecologic Oncology.2023;[Epub] CrossRef - Growing Teratoma Syndrome with Synchronous Gliomatosis Peritonei during Chemotherapy in Ovarian Immature Teratoma: A Case Report and Literature Review
Sijian Li, Na Su, Congwei Jia, Xinyue Zhang, Min Yin, Jiaxin Yang
Current Oncology.2022; 29(9): 6364. CrossRef - Extratesticular gliomatosis peritonei after mesenteric teratoma: a case report and literature review
Jiaqiang Li, Shoulin Li, Dong Xiao, Jiaming Song, Jianxiong Mao, Jianchun Yin
Journal of International Medical Research.2021;[Epub] CrossRef - Germ Cell Tumors of the Female Genital Tract
Elizabeth D. Euscher
Surgical Pathology Clinics.2019; 12(2): 621. CrossRef - Gliomatosis peritonei: a series of eight cases and review of the literature
Dan Wang, Cong-wei Jia, Rui-e Feng, Hong-hui Shi, Juan Sun
Journal of Ovarian Research.2016;[Epub] CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure

Fig. 1
| No. | Reference | Age (yr)/Sex | Involved site by endometriosis | Associated ovarian or peritoneal lesion | Increased serum oncoprotein |
Treatment | Outcome | |||
|---|---|---|---|---|---|---|---|---|---|---|
| AFP | CA125 | β-HCG | CA19-9 | |||||||
| 1 | Albukerk et al. (1979) [9] | 20/F | Cul-de-sac, uterus, pelvis, omentum | Immature teratoma (G2, 10 yr before), GP | ND | ND | ND | ND | ND | ND |
| 2 | Bassler et al. (1982) [10] | 22/F | Intestinal serosa, urinary bladder, Douglas pouch, omentum | Mat니re teratoma (12 yr before), GP | ND | ND | ND | ND | No further treatment | Recurrence of GP (1 yr) |
| 3 | Dworak et al. (1988) [11] | 9/F | Omentum, sacrouterine lesion | Immature teratoma (G1, 9 yr before), GP | WNL | WNL | WNL | ND | No further treatment | ND |
| 4 | Calder et al. (1994) [12] | 31/F | Douglas pouch, uterosacral ligament | Immature teratoma (G1, 20 yr before), GP | ND | ND | ND | ND | No further treatment | NR (4 yr) |
| 5 | Killeen et al. (1997) [13] | 30/F | Pelvic peritoneum, rectum, rectovaginal septum, uterus | Mature teratoma (G0, 16 yr before), GP | ND | ND | ND | ND | No further treatment | NR (5 yr) |
| 6 | Muller et al. (2002) [14] | 25/F | Peritoneum, greater omentum | Immature teratoma (G1, 4 yr before), GP | ND | ND | ND | ND | No further treatment | NR (5.5 yr) |
| 7 | Alexander et al. (2011) [15] | 59/F | Bilateral ovaries | Mature teratoma (23 yr before), GP, ovarian endometrioid adenocarcinoma and carcinoid, uterine endometrial mucinous adenocarcinoma | ND | ND | ND | ND | No further treatment for GP and endometriosis | ND |
| 8 | Kim et al. (present case) | 34/F | Cul-de-sac, omentum | Synchronous immature teratoma (G1), gliomatosis in the surface of the opposite ovary and uterine serosa, GP | ↑ | WNL | WNL | ↑ | No further treatment | NR (9 mo) |
| No | Reference | Age/Sex | Involved lymph node (grade of implant) | Plural or peritoneal effusion | Associated ovarian lesions or gliomatosis other than lymph node | Serum oncoprotein |
Treatment | Outcome | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AFP | CA125 | β-HCG | CA19-9 | ||||||||
| 1 | Benirschke et al. (1960) [1] | 18yr/F | Retroperitoneal, iliac, cervical, axillary (GO) | Ascites | Ovarian mature teratoma, GP | NA | NA | NA | NA | Chemoradiotherapy | Dead (8 mo) |
| 2 | Kourie and Roujeau (1966) [2] | 9yr/F | Mesenteric (G1) | Absent | Ovarian immature teratoma (G1), GP | ND | ND | ND | ND | No further treatment | NR (5 yr) |
| 3 | Robboy and Scully (1970) [3] | 9yr/F | Mesenteric (G1) | Absent | Ovarian immature teratoma (G1), omentum, GP | ND | ND | ND | ND | No further treatment | NR (5 yr) |
| 4 | Nagashima et al. (1974) [4] | 22 yr/F | Inguinal, mesenteric, mediastinal, cervical (malignant |
Ascites, pleural effusion | Ovarian immature teratoma (8 mo before) |
NA | NA | NA | NA | Chemotherapy | Dead (8 mo) |
| 5 | Perrone et al. (1986) [5] | 10mo/F | Para-aortic (GO) | Absent | Ovarian immature teratoma (G1), no GP | ↑ | WNL | WNL | WNL | No further treatment | NR (9 mo) |
| 6 | El Shafie et al. (1984) [6] | 12 yr/F | Omental (GO) | Ascites (1 L) | Ovarian teratoma, serosa of small and large intestine, appendiceal surface, GP | WNL | WNL | WNL | WNL | No further treatment | NR (5 yr) |
| 7 | Harms et al. (1989) [7] | 13 yr/F | Para-aortic (G3) | Absent | Ovarian immature teratoma (G1), GP, hepatic serosa | ND | ND | ND | ND | Chemotherapy | NR |
| 8 | KUan et al. (2005) [8] | 23 yr/F | Lymph node (GO) | Pleural effusion, ascites | Ovarian immature teratoma (G1), omentum, GP | ↑ | ↑ | ND | ↑ | Chemotherapy | ND |
| 9 | Kim et al. (present case) | 34 yr/F | Hypogastric (GO) | Ascites (200 mL) | Ovarian immature teratoma (G1), GP | ↑ | WNL | WNL | ↑ | No further treatment | NR (9 mo) |
AFP, alpha-fetoprotein; CA125, cancer antigen 125; β-HCG, beta-human chorionic gonadotropin; CA19-9, cancer antigen 19-9; F, female; G2, grade 2; GP, gliomatosis peritonei; ND, not described; G1, grade 1; WNL, within normal limits; G0, grade 0; NR, no recurrence.
AFP, alpha-fetoprotein; CA125, cancer antigen 125; β-HCG, beta-human chorionic gonadotropin; CA19-9, cancer antigen 19-9; F, female; G0, grade 0; GP, gliomatosis peritonei; NA, unavailable; G1, grade 1; ND, not described; NR, no recurrence; WNL, within normal limits; G3, grade 3. Grading is not described in the article.

 E-submission
E-submission