Search
- Page Path
- HOME > Search
Original Articles
- The combination of CDX2 expression status and tumor-infiltrating lymphocyte density as a prognostic factor in adjuvant FOLFOX-treated patients with stage III colorectal cancers
- Ji-Ae Lee, Hye Eun Park, Hye-Yeong Jin, Lingyan Jin, Seung Yeon Yoo, Nam-Yun Cho, Jeong Mo Bae, Jung Ho Kim, Gyeong Hoon Kang
- J Pathol Transl Med. 2025;59(1):50-59. Published online October 24, 2024
- DOI: https://doi.org/10.4132/jptm.2024.09.26
- 1,839 View
- 262 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
Colorectal carcinomas (CRCs) with caudal-type homeobox 2 (CDX2) loss are recognized to pursue an aggressive behavior but tend to be accompanied by a high density of tumor-infiltrating lymphocytes (TILs). However, little is known about whether there is an interplay between CDX2 loss and TIL density in the survival of patients with CRC.
Methods
Stage III CRC tissues were assessed for CDX2 loss using immunohistochemistry and analyzed for their densities of CD8 TILs in both intraepithelial (iTILs) and stromal areas using a machine learning-based analytic method.
Results
CDX2 loss was significantly associated with a higher density of CD8 TILs in both intraepithelial and stromal areas. Both CDX2 loss and a high CD8 iTIL density were found to be prognostic parameters and showed hazard ratios of 2.314 (1.050–5.100) and 0.378 (0.175–0.817), respectively, for cancer-specific survival. A subset of CRCs with retained CDX2 expression and a high density of CD8 iTILs showed the best clinical outcome (hazard ratio of 0.138 [0.023–0.826]), whereas a subset with CDX2 loss and a high density of CD8 iTILs exhibited the worst clinical outcome (15.781 [3.939–63.230]).
Conclusions
Altogether, a high density of CD8 iTILs did not make a difference in the survival of patients with CRC with CDX2 loss. The combination of CDX2 expression and intraepithelial CD8 TIL density was an independent prognostic marker in adjuvant chemotherapy-treated patients with stage III CRC.
- Senescent tumor cells in colorectal cancer are characterized by elevated enzymatic activity of complexes 1 and 2 in oxidative phosphorylation
- Jun Sang Shin, Tae-Gyu Kim, Young Hwa Kim, So Yeong Eom, So Hyun Park, Dong Hyun Lee, Tae Jun Park, Soon Sang Park, Jang-Hee Kim
- J Pathol Transl Med. 2023;57(6):305-314. Published online November 7, 2023
- DOI: https://doi.org/10.4132/jptm.2023.10.09
- 4,813 View
- 302 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
Cellular senescence is defined as an irreversible cell cycle arrest caused by various internal and external insults. While the metabolic dysfunction of senescent cells in normal tissue is relatively well-established, there is a lack of information regarding the metabolic features of senescent tumor cells.
Methods
Publicly available single-cell RNA-sequencing data from the GSE166555 and GSE178341 datasets were utilized to investigate the metabolic features of senescent tumor cells. To validate the single-cell RNA-sequencing data, we performed senescence-associated β-galactosidase (SA-β-Gal) staining to identify senescent tumor cells in fresh frozen colorectal cancer tissue. We also evaluated nicotinamide adenine dinucleotide dehydrogenase–tetrazolium reductase (NADH-TR) and succinate dehydrogenase (SDH) activity using enzyme histochemical methods and compared the staining with SA-β-Gal staining. MTT assay was performed to reveal the complex 1 activity of the respiratory chain in in-vitro senescence model.
Results
Single-cell RNA-sequencing data revealed an upregulation in the activity of complexes 1 and 2 in oxidative phosphorylation, despite overall mitochondrial dysfunction in senescent tumor cells. Both SA-β-Gal and enzyme histochemical staining using fresh frozen colorectal cancer tissues indicated a high correlation between SA-β-Gal positivity and NADH-TR/SDH staining positivity. MTT assay showed that senescent colorectal cancer cells exhibit higher absorbance in 600 nm wavelength.
Conclusions
Senescent tumor cells exhibit distinct metabolic features, characterized by upregulation of complexes 1 and 2 in the oxidative phosphorylation pathway. NADH-TR and SDH staining represent efficient methods for detecting senescent tumor cells in colorectal cancer. -
Citations
Citations to this article as recorded by- Cellular Aging and Senescence in Cancer: A Holistic Review of Cellular Fate Determinants
Muhammad Tufail, Yu-Qi Huang, Jia-Ju Hu, Jie Liang, Cai-Yun He, Wen-Dong Wan, Can-Hua Jiang, Hong Wu, Ning Li
Aging and disease.2024;[Epub] CrossRef - Real-time assessment of relative mitochondrial ATP synthesis response against inhibiting and stimulating substrates (MitoRAISE)
Eun Sol Chang, Kyoung Song, Ji-Young Song, Minjung Sung, Mi-Sook Lee, Jung Han Oh, Ji-Yeon Kim, Yeon Hee Park, Kyungsoo Jung, Yoon-La Choi
Cancer & Metabolism.2024;[Epub] CrossRef
- Cellular Aging and Senescence in Cancer: A Holistic Review of Cellular Fate Determinants
- Prognostic and clinicopathological significance of Fusobacterium nucleatum in colorectal cancer: a systemic review and meta-analysis
- Younghoon Kim, Nam Yun Cho, Gyeong Hoon Kang
- J Pathol Transl Med. 2022;56(3):144-151. Published online May 15, 2022
- DOI: https://doi.org/10.4132/jptm.2022.03.13
- 5,758 View
- 135 Download
- 8 Web of Science
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
Fusobacterium nucleatum has been identified to promote tumor progression in colorectal cancer (CRC). However, association between F. nucleatum and prognostic or clinicopathological features has been diverse among studies, which could be affected by type of biospecimen (formalin-fixed paraffin-embedded or fresh frozen [FF]).
Methods
Articles were systemically reviewed for studies that included the correlation between F. nucleatum and prognosis or clinicopathological features in CRC.
Results
Ten articles, eight studies with survival-related features involving 3,199 patients and nine studies with clinical features involving 2,655 patients, were eligible for the meta-analysis. Overall survival, disease-free survival, and cancer-specific survival were all associated with worse prognosis in F. nucleatum–high patients (p<.05). In subgroup analysis, only studies with FF tissues retained prognostic significance with F. nucleatum. In meta-analysis of clinicopathological variables, F. nucleatum level was associated with location within colon, pT category, MLH1 hypermethylation, microsatellite instability status, and BRAF mutation regardless of type of biospecimen. However, lymph node metastasis and KRAS mutation was only associated with F. nucleatum level in FF-based studies.
Conclusions
In conclusion, type of biospecimen could affect the role of F. nucleatum as a biomarker associated with clinicopathological features and prognosis. -
Citations
Citations to this article as recorded by- Unraveling the Role of Fusobacterium nucleatum in Colorectal Cancer: Molecular Mechanisms and Pathogenic Insights
Linda Galasso, Fabrizio Termite, Irene Mignini, Giorgio Esposto, Raffaele Borriello, Federica Vitale, Alberto Nicoletti, Mattia Paratore, Maria Elena Ainora, Antonio Gasbarrini, Maria Assunta Zocco
Cancers.2025; 17(3): 368. CrossRef - Intratumoural pks Escherichia coli is associated with risk of metachronous colorectal cancer and adenoma development in people with Lynch syndrome
Yen Lin Chu, Peter Georgeson, Mark Clendenning, Khalid Mahmood, Romy Walker, Julia Como, Sharelle Joseland, Susan G. Preston, Toni Rice, Brigid M. Lynch, Roger L. Milne, Melissa C. Southey, Graham G. Giles, Amanda I. Phipps, John L. Hopper, Aung K. Win, C
eBioMedicine.2025; 114: 105661. CrossRef - Fusobacterium nucleatum Enrichment in Colorectal Tumor Tissue: Associations With Tumor Characteristics and Survival Outcomes
Amanda I. Phipps, Courtney M. Hill, Genevieve Lin, Rachel C. Malen, Adriana M. Reedy, Orsalem Kahsai, Hamza Ammar, Keith Curtis, Ningxin Ma, Timothy W. Randolph, Jing Ma, Shuji Ogino, Polly A. Newcomb, Meredith AJ. Hullar
Gastro Hep Advances.2025; 4(6): 100644. CrossRef - Enhancing fibroblast–epithelial cell communications: Serpine2 as a key molecule in Fusobacterium nucleatum–promoted colon cancer
Xueke Li, Simin Luo, Yifang Jiang, Qiong Ma, Fengming You, Qixuan Kuang, Xi Fu, Chuan Zheng
Frontiers in Immunology.2025;[Epub] CrossRef - Intratumoral presence of the genotoxic gut bacteria pks+ E. coli, Enterotoxigenic Bacteroides fragilis, and Fusobacterium nucleatum and their association with clinicopathological and molecular features of colorectal cancer
Jihoon E. Joo, Yen Lin Chu, Peter Georgeson, Romy Walker, Khalid Mahmood, Mark Clendenning, Aaron L. Meyers, Julia Como, Sharelle Joseland, Susan G. Preston, Natalie Diepenhorst, Julie Toner, Danielle J. Ingle, Norelle L. Sherry, Andrew Metz, Brigid M. Ly
British Journal of Cancer.2024; 130(5): 728. CrossRef - The role of Fusobacterium nucleatum in cancer and its implications for clinical applications
Wanyi Luo, Juxi Han, Xian Peng, Xuedong Zhou, Tao Gong, Xin Zheng
Molecular Oral Microbiology.2024; 39(6): 417. CrossRef -
Fusobacterium nucleatum Load Correlates with KRAS Mutation and Sessile Serrated Pathogenesis in Colorectal Adenocarcinoma
Koki Takeda, Minoru Koi, Yoshiki Okita, Sija Sajibu, Temitope O. Keku, John M. Carethers
Cancer Research Communications.2023; 3(9): 1940. CrossRef - Tumour Colonisation of Parvimonas micra Is Associated with Decreased Survival in Colorectal Cancer Patients
Thyra Löwenmark, Anna Löfgren-Burström, Carl Zingmark, Ingrid Ljuslinder, Michael Dahlberg, Sofia Edin, Richard Palmqvist
Cancers.2022; 14(23): 5937. CrossRef
- Unraveling the Role of Fusobacterium nucleatum in Colorectal Cancer: Molecular Mechanisms and Pathogenic Insights
Case Study
- Colorectal adenocarcinoma with enteroblastic differentiation: diagnostic challenges of a rare case encountered in clinical practice
- Evi Abada, Ifeoma C. Anaya, Othuke Abada, Anthony Lebbos, Rafic Beydoun
- J Pathol Transl Med. 2022;56(2):97-102. Published online January 21, 2022
- DOI: https://doi.org/10.4132/jptm.2021.10.28
- 6,079 View
- 185 Download
- 9 Web of Science
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF - Colorectal adenocarcinoma with enteroblastic differentiation (CAED) is a rare subtype of colonic adenocarcinoma characterized by increased α-fetoprotein (AFP) production and the expression of at least one enteroblastic marker including AFP, glypican 3 (GPC3), or Spalt like transcription factor 4 (SALL4). We report a case of a 26-year-old female who presented with low back pain and constipation which persisted despite supportive measures. Imaging revealed multiple liver lesions and enlarged retroperitoneal nodes. Tumor markers including AFP were markedly elevated. On biopsy, samples from the liver revealed infiltrating glands lined by columnar-type epithelium with mostly eosinophilic granular to focally clear cytoplasm. By immunohistochemistry, the tumor showed immunoreactivity with AFP, hepatocyte antigen, GPC3, SALL4, CDX2, SATB2, and cytokeratin 20. A colonoscopy performed subsequently revealed a mass in the sigmoid colon and biopsy of this mass revealed a similar histology as that seen in the liver. A diagnosis of CAED was made, following the results of gene expression profiling by the tumor with next-generation sequencing which identified pathogenic variants in MUTYH, TP53, and KDM6A genes and therefore supported its colonic origin. Cases such as this underscores the use of ancillary diagnostic techniques in arriving at the correct diagnosis in lesions with overlapping clinicopathologic characteristics.
-
Citations
Citations to this article as recorded by- Exploring the Multifunctional Role of Alpha-Fetoprotein in Cancer Progression: Implications for Targeted Therapy in Hepatocellular Carcinoma and Beyond
Hyunjung Kim, Minji Jang, Eunmi Kim
International Journal of Molecular Sciences.2025; 26(10): 4863. CrossRef - SALL4 in gastrointestinal tract cancers: upstream and downstream regulatory mechanisms
Tairan Wang, Yan Jin, Mengyao Wang, Boya Chen, Jinyu Sun, Jiaying Zhang, Hui Yang, Xinyao Deng, Xingyue Cao, Lidong Wang, Yuanyuan Tang
Molecular Medicine.2024;[Epub] CrossRef - Gastric adenocarcinoma with enteroblastic differentiation in a
67-year-old man in Korea: a case report
Hae Rin Lee, Gwang Ha Kim, Dong Chan Joo, Moon Won Lee, Bong Eun Lee, Kyung Bin Kim
The Ewha Medical Journal.2024;[Epub] CrossRef - Colorectal adenocarcinoma with clear cell changes: immunohistological and molecular findings in three cases
Andreas Gocht, Carsten Heidel, Jutta Kirfel, Rita Vesce, Pamela Lazar-Karsten, Helen Pasternack, Madelaine Melzer, Phillip Hildebrand, Nicole Warkentin, Hendrik Schimmelpenning, Verena-Wilbeth Sailer
Virchows Archiv.2024; 485(3): 569. CrossRef - Ureteral Metastasis of Colonic Adenocarcinoma with Enteroblastic Differentiation: A Rare Case to be Distinguished from Clear Cell Adenocarcinoma of the Urinary Tract
Hiroshi Minato, Akane Yoshikawa, Sho Tsuyama, Kazuyoshi Katayanagi, Kengo Hayashi, Yusuke Sakimura, Hiroyuki Bando, Tomohiro Hori, Yosuke Kito
International Journal of Surgical Pathology.2023; 31(8): 1553. CrossRef - Beyond liver cancer, more application scenarios for alpha-fetoprotein in clinical practice
Chenyu Ma, Yuexinzi Jin, Yuhan Wang, Huaguo Xu, Jiexin Zhang
Frontiers in Oncology.2023;[Epub] CrossRef - AIEgens assisted label free DNA supersandwich immunoassay for ultrasensitive α-fetoprotein detection
Xiaowen Ou, Jingman Dai, Yiting Huang, Xiaoqin Xiong, Zhi Zheng, Xiaoding Lou, Fan Xia
Giant.2022; 11: 100110. CrossRef - Rectal carcinoma with dual differentiation toward enteroblastic and neuroendocrine features arising in a patient with ulcerative colitis: a case report
Takako Kihara, Ryuichi Kuwahara, Kurando Kusunoki, Tomohiro Minagawa, Yuki Horio, Motoi Uchino, Hiroki Ikeuchi, Seiichi Hirota
World Journal of Surgical Oncology.2022;[Epub] CrossRef
- Exploring the Multifunctional Role of Alpha-Fetoprotein in Cancer Progression: Implications for Targeted Therapy in Hepatocellular Carcinoma and Beyond
Original Articles
- MicroRNA-552 expression in colorectal cancer and its clinicopathological significance
- Joon Im, Soo Kyung Nam, Hye Seung Lee
- J Pathol Transl Med. 2021;55(2):125-131. Published online February 19, 2021
- DOI: https://doi.org/10.4132/jptm.2021.01.17
- 4,497 View
- 123 Download
- 3 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
MicroRNA-552 (miR-552) has been reported to correlate with the development and progression of various cancers, including colorectal cancer (CRC). This study aimed to investigate miR-552 expression in cancer tissue samples compared to normal mucosal tissue and its role as a diagnostic or prognostic marker in CRC patients.
Methods
Normal mucosal tissues and primary cancer tissues from 80 surgically resected CRC specimens were used. Quantitative real-time polymerase chain reaction was performed for miR-552 and U6 small nuclear RNA to analyze miR-552 expression and its clinicopathological significance. Immunohistochemistry for p53 and phosphatase and tension homolog (PTEN) was performed to evaluate their association with miR-552 expression.
Results
miR-552 expression was significantly higher in primary cancer tissues compared to normal mucosal tissues (p<.001). The expression level of miR552 was inversely correlated with that of PTEN (p=.068) and p53 (p=.004). Survival analysis showed that high miR-552 expression was associated with worse prognosis but this was not statistically significant (p=.255). However, patients with CRC having high miR-552 expression and loss of PTEN expression had significantly worse prognosis than others (p=.029).
Conclusions
Our results suggest that high miR-552 expression might be a potential diagnostic biomarker for CRC, and its combined analysis with PTEN expression can possibly be used as a prognostic marker. -
Citations
Citations to this article as recorded by- MicroRNAs involved in colorectal cancer, a rapid mini-systematic review
Sogol Shirzad, Majid Eterafi, Zeinab Karimi, Mahdi Barazesh
BMC Cancer.2025;[Epub] CrossRef - Diagnostic and Therapeutic Potential of Selected microRNAs in Colorectal Cancer: A Literature Review
Grzegorz Sychowski, Hanna Romanowicz, Wojciech Ciesielski, Piotr Hogendorf, Adam Durczyński, Beata Smolarz
Cancers.2025; 17(13): 2135. CrossRef - Blood miRNAs miR-549a, miR-552, and miR-592 serve as potential disease-specific panels to diagnose colorectal cancer
Soroush Akbar, Samaneh Mashreghi, Mohammad Reza Kalani, Akram Valanik, Farzaneh Ahmadi, Mahdi Aalikhani, Zahra Bazi
Heliyon.2024; 10(7): e28492. CrossRef - Integration of TE Induces Cancer Specific Alternative Splicing Events
Woo Ryung Kim, Eun Gyung Park, Yun Ju Lee, Woo Hyeon Bae, Du Hyeong Lee, Heui-Soo Kim
International Journal of Molecular Sciences.2022; 23(18): 10918. CrossRef
- MicroRNAs involved in colorectal cancer, a rapid mini-systematic review
- A comparative prognostic performance of definitions of Crohn-like lymphoid reaction in colorectal carcinoma
- Younghoon Kim, Jeong Mo Bae, Jung Ho Kim, Nam-Yun Cho, Gyeong Hoon Kang
- J Pathol Transl Med. 2021;55(1):53-59. Published online November 27, 2020
- DOI: https://doi.org/10.4132/jptm.2020.10.06
- 4,887 View
- 139 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
The prognostic potential of Crohn-like lymphoid reaction (CLR) in colorectal carcinoma (CRC) has been investigated through the assessment of different criteria.
Methods
The prognostic impact of CLR was investigated in 636 CRC patients to compare methods from previously published articles. These methods included CLR measured by number of lymphoid aggregates (LAs) (CLR count), LA size greater than or equal to 1 mm (CLR size), CLR density with a cutoff value of 0.38, and subjective criteria as defined by intense CLR.
Results
In univariate survival analysis, CLR-positive CRC as defined by the four aforementioned methods was associated with better overall survival (OS) (hazard ratio [HR], 0.463; 95% confidence interval [CI], 0.305 to 0.702; p <.001; HR, 0.656; 95% CI, 0.411 to 1.046; p=.077; HR, 0.363; 95% CI, 0.197 to 0.669; p=.001; and HR, 0.433; 95% CI, 0.271 to 0.690; p<.001, respectively) and disease-free survival (DFS) (HR, 0.411; 95% CI, 0.304 to 0.639; p<.001; HR, 0.528; 95% CI, 0.340 to 0.821; p=.004; HR, 0.382; 95% CI, 0.226 to 0.645, p=.004; and HR, 0.501; 95% CI, 0.339 to 0.741; p<.001, respectively) than CLR-negative CRC, regardless of criteria with the exception of OS for CLR density. In multivariate analysis, two objective criteria (CLR count and CLR density) and one subjective criterion (intense CLR) for defining CLR were considered independent prognostic factors of OS and DFS in CRC patients.
Conclusions
CLR has similar traits regardless of criteria, but CLR-positivity should be defined by objective criteria for better reproducibility and prognostic value.
Reviews
- Immune landscape and biomarkers for immuno-oncology in colorectal cancers
- Jeong Mo Bae, Seung-Yeon Yoo, Jung Ho Kim, Gyeong Hoon Kang
- J Pathol Transl Med. 2020;54(5):351-360. Published online June 26, 2020
- DOI: https://doi.org/10.4132/jptm.2020.05.15
- 8,134 View
- 319 Download
- 10 Web of Science
- 11 Crossref
-
 Abstract
Abstract
 PDF
PDF - Recent advances in immuno-oncology have increased understanding of the tumor immune microenvironment (TIME), and clinical trials for immune checkpoint inhibitor treatment have shown remission and/or durable response in certain proportions of patients stratified by predictive biomarkers. The TIME in colorectal cancer (CRC) was initially evaluated several decades ago. The prognostic value of the immune response to tumors, including tumor-infiltrating lymphocytes, peritumoral lymphoid reaction, and Crohn’s-like lymphoid reaction, has been well demonstrated. In this review, we describe the chronology of TIME research and review the up-to-date high-dimensional TIME landscape of CRC. We also summarize the clinical relevance of several biomarkers associated with immunotherapy in CRC, such as microsatellite instability, tumor mutational burden, POLE/POLD mutation, consensus molecular subtype, and programmed death-ligand 1 expression.
-
Citations
Citations to this article as recorded by- Targeting the “tumor microenvironment”: RNA-binding proteins in the spotlight in colorectal cancer therapy
Yiwei Zhang, Yujun Zhang, Jingjing Song, Xifu Cheng, Chulin Zhou, Shuo Huang, Wentao Zhao, Zhen Zong, Lingling Yang
International Immunopharmacology.2024; 131: 111876. CrossRef - Five decades of colorectal cancer pathology: The World and China
Maode Lai
Chinese Science Bulletin.2024;[Epub] CrossRef - T cell receptor clonotype in tumor microenvironment contributes to intratumoral signaling network in patients with colorectal cancer
In Hye Song, Seung-been Lee, Byung-Kwan Jeong, Jungwook Park, Honggeun Kim, GunHee Lee, Su Min Cha, Heejae Lee, Gyungyub Gong, Nak-Jung Kwon, Hee Jin Lee
Immunologic Research.2024; 72(5): 921. CrossRef - Identification of Key Immune and Cell Cycle Modules and Prognostic Genes for Glioma Patients through Transcriptome Analysis
Kaimin Guo, Jinna Yang, Ruonan Jiang, Xiaxia Ren, Peng Liu, Wenjia Wang, Shuiping Zhou, Xiaoguang Wang, Li Ma, Yunhui Hu
Pharmaceuticals.2024; 17(10): 1295. CrossRef - Unraveling the Role of Molecular Profiling in Predicting Treatment Response in Stage III Colorectal Cancer Patients: Insights from the IDEA International Study
Ippokratis Messaritakis, Eleni Psaroudaki, Konstantinos Vogiatzoglou, Maria Sfakianaki, Pantelis Topalis, Ioannis Iliopoulos, Dimitrios Mavroudis, John Tsiaoussis, Nikolaos Gouvas, Maria Tzardi, John Souglakos
Cancers.2023; 15(19): 4819. CrossRef - Biomarkers for Predicting Response to Personalized Immunotherapy in Gastric Cancer
Moonsik Kim, Ji Yun Jeong, An Na Seo
Diagnostics.2023; 13(17): 2782. CrossRef - Evaluation on Braking Stability of Autonomous Vehicles Running along Curved Sections Based on Asphalt Pavement Adhesion Properties
Binshuang Zheng, Xiaoming Huang, Junyao Tang, Jiaying Chen, Runmin Zhao, Zhengqiang Hong, Tao Tang, Meiling Han, Yang Yang
Journal of Advanced Transportation.2022; 2022: 1. CrossRef - Intratumoral spatial heterogeneity of tumor-infiltrating lymphocytes is a significant factor for precisely stratifying prognostic immune subgroups of microsatellite instability-high colorectal carcinomas
Minsun Jung, Ji Ae Lee, Seung-Yeon Yoo, Jeong Mo Bae, Gyeong Hoon Kang, Jung Ho Kim
Modern Pathology.2022; 35(12): 2011. CrossRef - Association of Tumor-Infiltrating Lymphocytes With Survival in Stages II and III Colorectal Cancer
Marina Vitorino, Inês Eiriz, Tiago C Tomás, Rodrigo Vicente, Ana Mendes, Ana Rita Freitas, Sofia Braga, Catarina Alves-Vale, Paula Borralho, André Ferreira, Luisa Leal da Costa
Cureus.2022;[Epub] CrossRef - Tumor Mutational Burden Predicting the Efficacy of Immune Checkpoint Inhibitors in Colorectal Cancer: A Systematic Review and Meta-Analysis
Yan Li, Yiqi Ma, Zijun Wu, Fanxin Zeng, Bin Song, Yanrong Zhang, Jinxing Li, Su Lui, Min Wu
Frontiers in Immunology.2021;[Epub] CrossRef - Genomic and transcriptomic characterization of heterogeneous immune subgroups of microsatellite instability-high colorectal cancers
Jung Ho Kim, Mi-Kyoung Seo, Ji Ae Lee, Seung-Yeon Yoo, Hyeon Jeong Oh, Hyundeok Kang, Nam-Yun Cho, Jeong Mo Bae, Gyeong Hoon Kang, Sangwoo Kim
Journal for ImmunoTherapy of Cancer.2021; 9(12): e003414. CrossRef
- Targeting the “tumor microenvironment”: RNA-binding proteins in the spotlight in colorectal cancer therapy
- Evolving pathologic concepts of serrated lesions of the colorectum
- Jung Ho Kim, Gyeong Hoon Kang
- J Pathol Transl Med. 2020;54(4):276-289. Published online June 26, 2020
- DOI: https://doi.org/10.4132/jptm.2020.04.15
- 13,039 View
- 802 Download
- 29 Web of Science
- 27 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Here, we provide an up-to-date review of the histopathology and molecular pathology of serrated colorectal lesions. First, we introduce the updated contents of the 2019 World Health Organization classification for serrated lesions. The sessile serrated lesion (SSL) is a new diagnostic terminology that replaces sessile serrated adenoma and sessile serrated polyp. The diagnostic criteria for SSL were revised to require only one unequivocal distorted serrated crypt, which is sufficient for diagnosis. Unclassified serrated adenomas have been included as a new category of serrated lesions. Second, we review ongoing issues concerning the morphology of serrated lesions. Minor morphologic variants with distinct molecular features were recently defined, including serrated tubulovillous adenoma, mucin-rich variant of traditional serrated adenoma (TSA), and superficially serrated adenoma. In addition to intestinal dysplasia and serrated dysplasia, minimal deviation dysplasia and not otherwise specified dysplasia were newly suggested as dysplasia subtypes of SSLs. Third, we summarize the molecular features of serrated lesions. The critical determinant of CpG island methylation development in SSLs is patient age. Interestingly, there may be ethnic differences in BRAF/KRAS mutation frequencies in SSLs. The molecular pathogenesis of TSAs is divided into KRAS and BRAF mutation pathways. SSLs with MLH1 methylation can progress into favorable prognostic microsatellite instability-positive (MSI+)/CpG island methylator phenotype-positive (CIMP+) carcinomas, whereas MLH1-unmethylated SSLs and BRAF-mutated TSAs can be precursors of poor-prognostic MSI−/CIMP+ carcinomas. Finally, based on our recent data, we propose an algorithm for stratifying risk subgroups of non-dysplastic SSLs.
-
Citations
Citations to this article as recorded by- Clinical and endoscopic characteristics of colorectal traditional serrated adenomas with dysplasia/adenocarcinoma in a Korean population
Ki-Hyun Kim, Eun Myung, Hyung Hoon Oh, Chan-Muk Im, Young-Eun Seo, Je-Seong Kim, Chae-June Lim, Ga-Ram You, Sung-Bum Cho, Wan-Sik Lee, Myung-Giun Noh, Kyung-Hwa Lee, Young-Eun Joo
World Journal of Gastrointestinal Oncology.2025;[Epub] CrossRef - Impact of AI-aided colonoscopy in clinical practice: a prospective randomised controlled trial
Johanna Schöler, Marko Alavanja, Thomas de Lange, Shunsuke Yamamoto, Per Hedenström, Jonas Varkey
BMJ Open Gastroenterology.2024; 11(1): e001247. CrossRef - The histologic features, molecular features, detection and management of serrated polyps: a review
Jin-Dong Wang, Guo-Shuai Xu, Xin-Long Hu, Wen-Qiang Li, Nan Yao, Fu-Zhou Han, Yin Zhang, Jun Qu
Frontiers in Oncology.2024;[Epub] CrossRef - Serrated polyps <10 mm cannot reliably be characterized by i-Scan without magnification at routine colonoscopy
Sabrina G.G. TESTONI, Chiara NOTARISTEFANO, Giuliano F. BONURA, Maria NAPOLITANO, Dario ESPOSITO, Edi VIALE, Lorella FANTI, Francesco AZZOLINI, Giulia M. CAVESTRO, PierAlberto TESTONI
Minerva Gastroenterology.2024;[Epub] CrossRef - Interobserver variability in the histopathological classification and grading of dysplasia in elevated colon lesions in the city of Lima
Guido Gallegos-Serruto, Aldo Gutiérrez, César Chian García, Isthvan Torres Perez
Revista de Gastroenterología del Perú.2024; 44(3): 239. CrossRef - Comparison of adenoma detection rate and proximal serrated polyp detection rate and their effect on post-colonoscopy colorectal cancer mortality in screening patients
Jasmin Zessner-Spitzenberg, Elisabeth Waldmann, Lena Jiricka, Lisa-Maria Rockenbauer, Anna Hinterberger, Jeremy Cook, Arno Asaturi, Aleksandra Szymanska, Barbara Majcher, Michael Trauner, Monika Ferlitsch
Endoscopy.2023; 55(05): 434. CrossRef - The yield of dysplasia and serrated lesions in a single-centre tertiary inflammatory bowel disease cohort
Fiona Yeaman, Lena Thin
Therapeutic Advances in Gastroenterology.2023;[Epub] CrossRef -
The BEETS (JACCRO CC-18) Trial: An Observational and Translational Study of
BRAF
-Mutated Metastatic Colorectal Cancer
Chiaki Inagaki, Ryo Matoba, Satoshi Yuki, Manabu Shiozawa, Akihito Tsuji, Eisuke Inoue, Kei Muro, Wataru Ichikawa, Masashi Fujii, Yu Sunakawa
Future Oncology.2023; 19(17): 1165. CrossRef - A retrospective analysis of the histology of resected polyps and colonoscopy quality parameters in Belgium
E Macken, S Van Dongen, G Van Hal
Acta Gastro Enterologica Belgica.2023; 86(2): 277. CrossRef - Prognostic Biomarkers of Cell Proliferation in Colorectal Cancer (CRC): From Immunohistochemistry to Molecular Biology Techniques
Aldona Kasprzak
Cancers.2023; 15(18): 4570. CrossRef - Assimilating Epigenetics and Transcriptomics for the Identification
of Prognostic Novel Biomarkers and Imminent Targets in
Colorectal Carcinoma with Therapeutic Potential
Suman Kumar Ray, Sukhes Mukherjee
Current Molecular Medicine.2023; 23(8): 784. CrossRef - Multitarget Stool RNA Test for Colorectal Cancer Screening
Erica K. Barnell, Elizabeth M. Wurtzler, Julie La Rocca, Thomas Fitzgerald, Jessica Petrone, Yansheng Hao, Yiming Kang, Faith L. Holmes, David A. Lieberman
JAMA.2023; 330(18): 1760. CrossRef - Microbiome in Colonic Carcinogenesis
Jun Sun, Yinglin Xia
Comprehensive Physiology.2023; 13(3): 4685. CrossRef - Impact of comprehensive optical diagnosis training using Workgroup serrAted polypS and Polyposis classification on detection of adenoma and sessile serrated lesion
Jooyoung Lee, Jung Ho Bae, Su Jin Chung, Hae Yeon Kang, Seung Joo Kang, Min‐Sun Kwak, Ji Yeon Seo, Ji Hyun Song, Sun Young Yang, Jong In Yang, Seon Hee Lim, Jeong Yoon Yim, Joo Hyun Lim, Goh Eun Chung, Eun Hyo Jin, Ji Min Choi, Yoo Min Han, Joo Sung Kim
Digestive Endoscopy.2022; 34(1): 180. CrossRef - Clinicopathological and molecular analyses of hyperplastic lesions including microvesicular variant and goblet cell rich variant hyperplastic polyps and hyperplastic nodules—Hyperplastic nodule is an independent histological entity
Noriyuki Uesugi, Yoichi Ajioka, Tomio Arai, Yoshihito Tanaka, Tamotsu Sugai
Pathology International.2022; 72(2): 128. CrossRef - Comprehensive clinicopathologic, molecular, and immunologic characterization of colorectal carcinomas with loss of three intestinal markers, CDX2, SATB2, and KRT20
Ji Ae Lee, Mi-Kyoung Seo, Seung-Yeon Yoo, Nam-Yun Cho, Yoonjin Kwak, Kyoungbun Lee, Jung Ho Kim, Gyeong Hoon Kang
Virchows Archiv.2022; 480(3): 543. CrossRef - Serrated Colorectal Lesions: An Up-to-Date Review from Histological Pattern to Molecular Pathogenesis
Martino Mezzapesa, Giuseppe Losurdo, Francesca Celiberto, Salvatore Rizzi, Antonio d’Amati, Domenico Piscitelli, Enzo Ierardi, Alfredo Di Leo
International Journal of Molecular Sciences.2022; 23(8): 4461. CrossRef - Arterial stiffness is associated with high-risk colorectal adenomas and serrated lesions: A cross-sectional study in a Taiwanese population
Hung-Yu Chen, Wen-Huang Lee, Hung-Lung Hsu, Yu-Tsung Chou, Fei-Lin Su, I-Hsuan Wu, Ting-Hsing Chao
Journal of Cardiology.2022; 80(2): 139. CrossRef - Morphological and molecular characterization of colorectal sessile serrated lesions with dysplasia
Filippo Cappello, Valentina Angerilli, Luca Dal Santo, Giada Munari, Marianna Sabbadin, Marcello Lo Mele, Gianmaria Pennelli, Claudio Luchini, Paola Parente, Stefano Lazzi, Matteo Fassan
Pathology - Research and Practice.2022; 240: 154214. CrossRef - Serrated polyposis: an overview
Jonathan Fawkes
Gastrointestinal Nursing.2022; 20(9): 24. CrossRef - Sessile serrated lesion presenting as large pedunculated polyp in the rectum: A case report
Shin Ju Oh, Jung-Wook Kim, Chi Hyuk Oh
Medicine.2022; 101(51): e32287. CrossRef - WHICH LESIONS ARE AT HIGHER RISK OF DEVELOPING COLORECTAL CARCINOMAS: SUPERFICIALLY ELEVATED SERRATED LESIONS OR DEPRESSED LESIONS?
Artur Adolfo PARADA, Filadelfio Euclydes VENCO, Miguel Reynaldo VARCA-NETO, Roberto EL IBRAHIM, Paula Bechara POLETTI, Helcio Pedrosa BRITO, Heloisa de Fátima SARE, Osvaldo MALAFAIA
ABCD. Arquivos Brasileiros de Cirurgia Digestiva (São Paulo).2022;[Epub] CrossRef - WNT5a in Colorectal Cancer: Research Progress and Challenges
Guangshun Sun, Liangliang Wu, Guoqiang Sun, Xuesong Shi, Hongyong Cao, Weiwei Tang
Cancer Management and Research.2021; Volume 13: 2483. CrossRef - Endoscopic diagnosis for colorectal sessile serrated lesions
Toshihiro Nishizawa, Shuntaro Yoshida, Akira Toyoshima, Tomoharu Yamada, Yoshiki Sakaguchi, Taiga Irako, Hirotoshi Ebinuma, Takanori Kanai, Kazuhiko Koike, Osamu Toyoshima
World Journal of Gastroenterology.2021; 27(13): 1321. CrossRef - NTRK oncogenic fusions are exclusively associated with the serrated neoplasia pathway in the colorectum and begin to occur in sessile serrated lesions
Jung Ho Kim, Jeong Hoon Hong, Yoon‐La Choi, Ji Ae Lee, Mi‐kyoung Seo, Mi‐Sook Lee, Sung Bin An, Min Jung Sung, Nam‐Yun Cho, Sung‐Su Kim, Young Kee Shin, Sangwoo Kim, Gyeong Hoon Kang
The Journal of Pathology.2021; 255(4): 399. CrossRef - Differential pre-malignant programs and microenvironment chart distinct paths to malignancy in human colorectal polyps
Bob Chen, Cherie’ R. Scurrah, Eliot T. McKinley, Alan J. Simmons, Marisol A. Ramirez-Solano, Xiangzhu Zhu, Nicholas O. Markham, Cody N. Heiser, Paige N. Vega, Andrea Rolong, Hyeyon Kim, Quanhu Sheng, Julia L. Drewes, Yuan Zhou, Austin N. Southard-Smith, Y
Cell.2021; 184(26): 6262. CrossRef - Molecular Insights Into Colorectal Carcinoma
Domenika Ortiz Requena, Monica Garcia-Buitrago
Archives of Medical Research.2020; 51(8): 839. CrossRef
- Clinical and endoscopic characteristics of colorectal traditional serrated adenomas with dysplasia/adenocarcinoma in a Korean population
Original Article
- Colorectal epithelial neoplasm associated with gut-associated lymphoid tissue
- Yo Han Jeon, Ji Hyun Ahn, Hee Kyung Chang
- J Pathol Transl Med. 2020;54(2):135-145. Published online January 29, 2020
- DOI: https://doi.org/10.4132/jptm.2019.11.06
- 7,710 View
- 245 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Colorectal epithelial neoplasm extending into the submucosal gut-associated lymphoid tissue (GALT) can cause difficulties in the differential diagnosis. Regarding GALT-associated epithelial neoplasms, a few studies favor the term “GALT carcinoma” while other studies have mentioned the term “GALT-associated pseudoinvasion/epithelial misplacement (PEM)”.
Methods
The clinicopathologic characteristics of 11 cases of colorectal epithelial neoplasm associated with submucosal GALT diagnosed via endoscopic submucosal dissection were studied.
Results
Eight cases (72.7%) were in males. The median age was 59 years, and age ranged from 53 to 73. All cases had a submucosal tumor component more compatible with GALT-associated PEM. Eight cases (72.7%) were located in the right colon. Ten cases (90.9%) had a non-protruding endoscopic appearance. Nine cases (81.8%) showed continuity between the submucosal and surface adenomatous components. Nine cases showed (81.8%) focal defects or discontinuation of the muscularis mucosae adjacent to the submucosal GALT. No case showed hemosiderin deposits in the submucosa or desmoplastic reaction. No case showed single tumor cells or small clusters of tumor cells in the submucosal GALT. Seven cases (63.6%) showed goblet cells in the submucosa. No cases showed oncocytic columnar cells lining submucosal glands.
Conclusions
Our experience suggests that pathologists should be aware of the differential diagnosis of GALT-associated submucosal extension by colorectal adenomatous neoplasm. Further studies are needed to validate classification of GALT-associated epithelial neoplasms. -
Citations
Citations to this article as recorded by- Family adenomatous polyposis come across dome type adenocarcinoma: a case report and literature review
Ying-Ying Chang, Xiao-Long Zhang, Yao-Hui Wang, Ting-Sheng Ling
Diagnostic Pathology.2025;[Epub] CrossRef - Radiation-induced injury and the gut microbiota: insights from a microbial perspective
Qiaoli Wang, Guoqiang Xu, Ouying Yan, Shang Wang, Xin Wang
Therapeutic Advances in Gastroenterology.2025;[Epub] CrossRef
- Family adenomatous polyposis come across dome type adenocarcinoma: a case report and literature review
Review
- Standardized Pathology Report for Colorectal Cancer, 2nd Edition
- Baek-hui Kim, Joon Mee Kim, Gyeong Hoon Kang, Hee Jin Chang, Dong Wook Kang, Jung Ho Kim, Jeong Mo Bae, An Na Seo, Ho Sung Park, Yun Kyung Kang, Kyung-Hwa Lee, Mee Yon Cho, In-Gu Do, Hye Seung Lee, Hee Kyung Chang, Do Youn Park, Hyo Jeong Kang, Jin Hee Sohn, Mee Soo Chang, Eun Sun Jung, So-Young Jin, Eunsil Yu, Hye Seung Han, Youn Wha Kim
- J Pathol Transl Med. 2020;54(1):1-19. Published online November 13, 2019
- DOI: https://doi.org/10.4132/jptm.2019.09.28
- 22,605 View
- 1,251 Download
- 42 Web of Science
- 35 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - The first edition of the ‘Standardized Pathology Report for Colorectal Cancer,’ which was developed by the Gastrointestinal Pathology Study Group (GIP) of the Korean Society of Pathologists, was published 13 years ago. Meanwhile, there have been many changes in the pathologic diagnosis of colorectal cancer (CRC), pathologic findings included in the pathology report, and immunohistochemical and molecular pathology required for the diagnosis and treatment of colorectal cancer. In order to reflect these changes, we (GIP) decided to make the second edition of the report. The purpose of this standardized pathology report is to provide a practical protocol for Korean pathologists, which could help diagnose and treat CRC patients. This report consists of “standard data elements” and “conditional data elements.” Basic pathologic findings and parts necessary for prognostication of CRC patients are classified as “standard data elements,” while other prognostic factors and factors related to adjuvant therapy are classified as “conditional data elements” so that each institution could select the contents according to the characteristics of the institution. The Korean version is also provided separately so that Korean pathologists can easily understand and use this report. We hope that this report will be helpful in the daily practice of CRC diagnosis.
-
Citations
Citations to this article as recorded by- Diagnostic accuracy and pitfalls of MRI for restaging locally advanced rectal cancer in patients following anti-PD1 therapy plus neoadjuvant chemoradiotherapy: a multicenter study
Lixue Xu, Liting Sun, Yuhuan Fu, Guangyong Chen, Hongwei Yao, Zhenchang Wang, Zhenghan Yang, Jie Zhang
Abdominal Radiology.2025;[Epub] CrossRef - Additional staining for lymphovascular invasion is associated with increased estimation of lymph node metastasis in patients with T1 colorectal cancer: Systematic review and meta‐analysis
Jun Watanabe, Katsuro Ichimasa, Yuki Kataoka, Atsushi Miki, Hidehiro Someko, Munenori Honda, Makiko Tahara, Takeshi Yamashina, Khay Guan Yeoh, Shigeo Kawai, Kazuhiko Kotani, Naohiro Sata
Digestive Endoscopy.2024; 36(5): 533. CrossRef - The use of core descriptors from the ENiGMA code study in recent literature: a systematic review
Saher‐Zahra Khan, Andrea Arline, Kate M. Williams, Matthew J. Lee, Emily Steinhagen, Sharon L. Stein
Colorectal Disease.2024; 26(3): 428. CrossRef - Efficacy and safety of PD-1 blockade plus long-course chemoradiotherapy in locally advanced rectal cancer (NECTAR): a multi-center phase 2 study
Zhengyang Yang, Jiale Gao, Jianyong Zheng, Jiagang Han, Ang Li, Gang Liu, Yi Sun, Jie Zhang, Guangyong Chen, Rui Xu, Xiao Zhang, Yishan Liu, Zhigang Bai, Wei Deng, Wei He, Hongwei Yao, Zhongtao Zhang
Signal Transduction and Targeted Therapy.2024;[Epub] CrossRef - Diagnostic Accuracy of Highest-Grade or Predominant Histological Differentiation of T1 Colorectal Cancer in Predicting Lymph Node Metastasis: A Systematic Review and Meta-Analysis
Jun Watanabe, Katsuro Ichimasa, Yuki Kataoka, Shoko Miyahara, Atsushi Miki, Khay Guan Yeoh, Shigeo Kawai, Fernando Martínez de Juan, Isidro Machado, Kazuhiko Kotani, Naohiro Sata
Clinical and Translational Gastroenterology.2024; 15(3): e00673. CrossRef - Comparative evaluation of CT and MRI in the preoperative staging of colon cancer
Effrosyni Bompou, Aikaterini Vassiou, Ioannis Baloyiannis, Konstantinos Perivoliotis, Ioannis Fezoulidis, George Tzovaras
Scientific Reports.2024;[Epub] CrossRef - Pathologic Implications of Magnetic Resonance Imaging-detected Extramural Venous Invasion of Rectal Cancer
Hyun Gu Lee, Chan Wook Kim, Jong Keon Jang, Seong Ho Park, Young Il Kim, Jong Lyul Lee, Yong Sik Yoon, In Ja Park, Seok-Byung Lim, Chang Sik Yu, Jin Cheon Kim
Clinical Colorectal Cancer.2023; 22(1): 129. CrossRef - A standardized pathology report for gastric cancer: 2nd edition
Young Soo Park, Myeong-Cherl Kook, Baek-hui Kim, Hye Seung Lee, Dong-Wook Kang, Mi-Jin Gu, Ok Ran Shin, Younghee Choi, Wonae Lee, Hyunki Kim, In Hye Song, Kyoung-Mee Kim, Hee Sung Kim, Guhyun Kang, Do Youn Park, So-Young Jin, Joon Mee Kim, Yoon Jung Choi,
Journal of Pathology and Translational Medicine.2023; 57(1): 1. CrossRef - IGFL2-AS1, a Long Non-Coding RNA, Is Associated with Radioresistance in Colorectal Cancer
Jeeyong Lee, Da Yeon Kim, Younjoo Kim, Ui Sup Shin, Kwang Seok Kim, Eun Ju Kim
International Journal of Molecular Sciences.2023; 24(2): 978. CrossRef - A Standardized Pathology Report for Gastric Cancer: 2nd Edition
Young Soo Park, Myeong-Cherl Kook, Baek-hui Kim, Hye Seung Lee, Dong-Wook Kang, Mi-Jin Gu, Ok Ran Shin, Younghee Choi, Wonae Lee, Hyunki Kim, In Hye Song, Kyoung-Mee Kim, Hee Sung Kim, Guhyun Kang, Do Youn Park, So-Young Jin, Joon Mee Kim, Yoon Jung Choi,
Journal of Gastric Cancer.2023; 23(1): 107. CrossRef - Incremental Detection Rate of Dysplasia and Sessile Serrated Polyps/Adenomas Using Narrow-Band Imaging and Dye Spray Chromoendoscopy in Addition to High-Definition Endoscopy in Patients with Long-Standing Extensive Ulcerative Colitis: Segmental Tandem End
Ji Eun Kim, Chang Wan Choi, Sung Noh Hong, Joo Hye Song, Eun Ran Kim, Dong Kyung Chang, Young-Ho Kim
Diagnostics.2023; 13(3): 516. CrossRef - Prognostic Impact of Extramural Lymphatic, Vascular, and Perineural Invasion in Stage II Colon Cancer: A Comparison With Intramural Invasion
Sang Sik Cho, Ji Won Park, Gyeong Hoon Kang, Jung Ho Kim, Jeong Mo Bae, Sae-Won Han, Tae-You Kim, Min Jung Kim, Seung-Bum Ryoo, Seung-Yong Jeong, Kyu Joo Park
Diseases of the Colon & Rectum.2023; 66(3): 366. CrossRef - Towards targeted colorectal cancer biopsy based on tissue morphology assessment by compression optical coherence elastography
Anton A. Plekhanov, Marina A. Sirotkina, Ekaterina V. Gubarkova, Elena B. Kiseleva, Alexander A. Sovetsky, Maria M. Karabut, Vladimir E. Zagainov, Sergey S. Kuznetsov, Anna V. Maslennikova, Elena V. Zagaynova, Vladimir Y. Zaitsev, Natalia D. Gladkova
Frontiers in Oncology.2023;[Epub] CrossRef - Is High-Grade Tumor Budding an Independent Prognostic Factor in Stage II Colon Cancer?
Jung Kyong Shin, Yoon Ah Park, Jung Wook Huh, Seong Hyeon Yun, Hee Cheol Kim, Woo Yong Lee, Seok Hyung Kim, Sang Yun Ha, Yong Beom Cho
Diseases of the Colon & Rectum.2023; 66(8): e801. CrossRef - Detection of Human cytomegalovirus UL55 Gene and IE/E Protein Expression in Colorectal Cancer Patients in Egypt
May Raouf, Ahmed A. Sabry, Mahinour A. Ragab, Samar El Achy, Amira Amer
BMC Cancer.2023;[Epub] CrossRef - Polo-like kinase 4 as a potential predictive biomarker of chemoradioresistance in locally advanced rectal cancer
Hyunseung Oh, Soon Gu Kim, Sung Uk Bae, Sang Jun Byun, Shin Kim, Jae-Ho Lee, Ilseon Hwang, Sun Young Kwon, Hye Won Lee
Journal of Pathology and Translational Medicine.2022; 56(1): 40. CrossRef - A Prediction Model for Tumor Recurrence in Stage II–III Colorectal Cancer Patients: From a Machine Learning Model to Genomic Profiling
Po-Chuan Chen, Yu-Min Yeh, Bo-Wen Lin, Ren-Hao Chan, Pei-Fang Su, Yi-Chia Liu, Chung-Ta Lee, Shang-Hung Chen, Peng-Chan Lin
Biomedicines.2022; 10(2): 340. CrossRef - Rationale and design of a prospective, multicenter, phase II clinical trial of safety and efficacy evaluation of long course neoadjuvant chemoradiotherapy plus tislelizumab followed by total mesorectal excision for locally advanced rectal cancer (NCRT-PD1
Zhengyang Yang, Xiao Zhang, Jie Zhang, Jiale Gao, Zhigang Bai, Wei Deng, Guangyong Chen, Yongbo An, Yishan Liu, Qi Wei, Jiagang Han, Ang Li, Gang Liu, Yi Sun, Dalu Kong, Hongwei Yao, Zhongtao Zhang
BMC Cancer.2022;[Epub] CrossRef - Potential of DEK proto‑oncogene as a prognostic biomarker for colorectal cancer: An evidence‑based review
Muhammad Habiburrahman, Muhammad Wardoyo, Stefanus Sutopo, Nur Rahadiani
Molecular and Clinical Oncology.2022;[Epub] CrossRef - Reproducibility and Feasibility of Classification and National Guidelines for Histological Diagnosis of Canine Mammary Gland Tumours: A Multi-Institutional Ring Study
Serenella Papparella, Maria Crescio, Valeria Baldassarre, Barbara Brunetti, Giovanni Burrai, Cristiano Cocumelli, Valeria Grieco, Selina Iussich, Lorella Maniscalco, Francesca Mariotti, Francesca Millanta, Orlando Paciello, Roberta Rasotto, Mariarita Roma
Veterinary Sciences.2022; 9(7): 357. CrossRef - Composite scoring system and optimal tumor budding cut-off number for estimating lymph node metastasis in submucosal colorectal cancer
Jeong-ki Kim, Ye-Young Rhee, Jeong Mo Bae, Jung Ho Kim, Seong-Joon Koh, Hyun Jung Lee, Jong Pil Im, Min Jung Kim, Seung-Bum Ryoo, Seung-Yong Jeong, Kyu Joo Park, Ji Won Park, Gyeong Hoon Kang
BMC Cancer.2022;[Epub] CrossRef - Automated Hybrid Model for Detecting Perineural Invasion in the Histology of Colorectal Cancer
Jiyoon Jung, Eunsu Kim, Hyeseong Lee, Sung Hak Lee, Sangjeong Ahn
Applied Sciences.2022; 12(18): 9159. CrossRef - Clinical Implication of Perineural and Lymphovascular Invasion in Rectal Cancer Patients Who Underwent Surgery After Preoperative Chemoradiotherapy
Young Il Kim, Chan Wook Kim, Jong Hoon Kim, Jihun Kim, Jun-Soo Ro, Jong Lyul Lee, Yong Sik Yoon, In Ja Park, Seok-Byung Lim, Chang Sik Yu, Jin Cheon Kim
Diseases of the Colon & Rectum.2022; 65(11): 1325. CrossRef - Molecular Pathology of Gastric Cancer
Moonsik Kim, An Na Seo
Journal of Gastric Cancer.2022; 22(4): 264. CrossRef - Selective approach to arterial ligation in radical sigmoid colon cancer surgery with D3 lymph node dissection: A multicenter comparative study
Sergey Efetov, Albina Zubayraeva, Cüneyt Kayaalp, Alisa Minenkova, Yusuf Bağ, Aftandil Alekberzade, Petr Tsarkov
Turkish Journal of Surgery.2022; 38(4): 382. CrossRef - Evaluation of lncRNA FOXD2-AS1 Expression as a Diagnostic Biomarker in Colorectal Cancer
Hooman Shalmashi, Sahar Safaei, Dariush Shanehbandi, Milad Asadi, Soghra Bornehdeli, Abdolreza Mehdi Navaz
Reports of Biochemistry and Molecular Biology.2022; 11(3): 471. CrossRef - Improvement in the Assessment of Response to Preoperative Chemoradiotherapy for Rectal Cancer Using Magnetic Resonance Imaging and a Multigene Biomarker
Eunhae Cho, Sung Woo Jung, In Ja Park, Jong Keon Jang, Seong Ho Park, Seung-Mo Hong, Jong Lyul Lee, Chan Wook Kim, Yong Sik Yoon, Seok-Byung Lim, Chang Sik Yu, Jin Cheon Kim
Cancers.2021; 13(14): 3480. CrossRef - Addition of V-Stage to Conventional TNM Staging to Create the TNVM Staging System for Accurate Prediction of Prognosis in Colon Cancer: A Multi-Institutional Retrospective Cohort Study
Jung Hoon Bae, Ji Hoon Kim, Jaeim Lee, Bong-Hyeon Kye, Sang Chul Lee, In Kyu Lee, Won Kyung Kang, Hyeon-Min Cho, Yoon Suk Lee
Biomedicines.2021; 9(8): 888. CrossRef - Gene Expression Profiles Associated with Radio-Responsiveness in Locally Advanced Rectal Cancer
Jeeyong Lee, Junhye Kwon, DaYeon Kim, Misun Park, KwangSeok Kim, InHwa Bae, Hyunkyung Kim, JoonSeog Kong, Younjoo Kim, UiSup Shin, EunJu Kim
Biology.2021; 10(6): 500. CrossRef - A Patient-Derived Organoid-Based Radiosensitivity Model for the Prediction of Radiation Responses in Patients with Rectal Cancer
Misun Park, Junhye Kwon, Joonseog Kong, Sun Mi Moon, Sangsik Cho, Ki Young Yang, Won Il Jang, Mi Sook Kim, Younjoo Kim, Ui Sup Shin
Cancers.2021; 13(15): 3760. CrossRef - Comparison between Local Excision and Radical Resection for the Treatment of Rectal Cancer in ypT0-1 Patients: An Analysis of the Clinicopathological Factors and Survival Rates
Soo Young Oh, In Ja Park, Young IL Kim, Jong-Lyul Lee, Chan Wook Kim, Yong Sik Yoon, Seok-Byung Lim, Chang Sik Yu, Jin Cheon Kim
Cancers.2021; 13(19): 4823. CrossRef - Comparison of Vascular Invasion With Lymph Node Metastasis as a Prognostic Factor in Stage I-III Colon Cancer: An Observational Cohort Study
Jung Hoon Bae, Ji Hoon Kim, Bong-Hyeon Kye, Abdullah Al-Sawat, Chul Seung Lee, Seung-Rim Han, In Kyu Lee, Sung Hak Lee, Yoon Suk Lee
Frontiers in Surgery.2021;[Epub] CrossRef - Clinicopathological significance of Ki67 expression in colorectal cancer
Jing Li, Zhi-ye Liu, Hai-bo Yu, Qing Xue, Wen-jie He, Hai-tao Yu
Medicine.2020; 99(20): e20136. CrossRef - Lateral lymph node and its association with distant recurrence in rectal cancer: A clue of systemic disease
Young Il Kim, Jong Keon Jang, In Ja Park, Seong Ho Park, Jong Beom Kim, Jin-Hong Park, Tae Won Kim, Jun-Soo Ro, Seok-Byung Lim, Chang Sik Yu, Jin Cheon Kim
Surgical Oncology.2020; 35: 174. CrossRef - Transformation of Pathology Reports Into the Common Data Model With Oncology Module: Use Case for Colon Cancer
Borim Ryu, Eunsil Yoon, Seok Kim, Sejoon Lee, Hyunyoung Baek, Soyoung Yi, Hee Young Na, Ji-Won Kim, Rong-Min Baek, Hee Hwang, Sooyoung Yoo
Journal of Medical Internet Research.2020; 22(12): e18526. CrossRef
- Diagnostic accuracy and pitfalls of MRI for restaging locally advanced rectal cancer in patients following anti-PD1 therapy plus neoadjuvant chemoradiotherapy: a multicenter study
Original Articles
- Clinicopathological Characterization and Prognostic Implication of SMAD4 Expression in Colorectal Carcinoma
- Seung-Yeon Yoo, Ji-Ae Lee, Yunjoo Shin, Nam-Yun Cho, Jeong Mo Bae, Gyeong Hoon Kang
- J Pathol Transl Med. 2019;53(5):289-297. Published online June 24, 2019
- DOI: https://doi.org/10.4132/jptm.2019.06.07
- 8,043 View
- 161 Download
- 8 Web of Science
- 9 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
SMAD family member 4 (SMAD4) has gained attention as a promising prognostic factor of colorectal cancer (CRC) as well as a key molecule to understand the tumorigenesis and progression of CRC.
Methods
We retrospectively analyzed 1,281 CRC cases immunohistochemically for their expression status of SMAD4, and correlated this status with clinicopathologic and molecular features of CRCs.
Results
A loss of nuclear SMAD4 was significantly associated with frequent lymphovascular and perineural invasion, tumor budding, fewer tumor-infiltrating lymphocytes, higher pT and pN category, and frequent distant metastasis. In contrast, tumors overexpressing SMAD4 showed a significant association with sporadic microsatellite instability. After adjustment for TNM stage, tumor differentiation, adjuvant chemotherapy, and lymphovascular invasion, the loss of SMAD4 was found to be an independent prognostic factor for worse 5-year progression-free survival (hazard ratio [HR], 1.27; 95% confidence interval [CI], 1.01 to 1.60; p=.042) and 7-year cancerspecific survival (HR, 1.45; 95% CI, 1.06 to 1.99; p=.022).
Conclusions
We confirmed the value of determining the loss of SMAD4 immunohistochemically as an independent prognostic factor for CRC in general. In addition, we identified some histologic and molecular features that might be clues to elucidate the role of SMAD4 in colorectal tumorigenesis and progression. -
Citations
Citations to this article as recorded by- Association between the expression of epithelial–mesenchymal transition (EMT)-related markers and oncologic outcomes of colorectal cancer
Mona Hany Emile, Sameh Hany Emile, Amr Awad El-Karef, Mohamed Awad Ebrahim, Ibrahim Eldosoky Mohammed, Dina Abdallah Ibrahim
Updates in Surgery.2024; 76(6): 2181. CrossRef - TGF-β and SMAD2/4 Expression in Nonmetastatic and Metastatic Colorectal Cancer Patients
Ainul Mardiah, Hendra Susanto, Sri Rahayu Lestari, A. Taufiq, H. Susanto, H. Nur, M. Diantoro, M. Aziz, N.A.N.N. Malek
BIO Web of Conferences.2024; 117: 01001. CrossRef - Unraveling Resistance to Immunotherapy in MSI-High Colorectal Cancer
Ronald Heregger, Florian Huemer, Markus Steiner, Alejandra Gonzalez-Martinez, Richard Greil, Lukas Weiss
Cancers.2023; 15(20): 5090. CrossRef - Association of β-Catenin, APC, SMAD3/4, Tp53, and Cyclin D1 Genes in Colorectal Cancer: A Systematic Review and Meta-Analysis
Hongfeng Yan, Fuquan Jiang, Jianwu Yang, Ying-Kun Xu
Genetics Research.2022; 2022: 1. CrossRef - Comprehensive genetic features of gastric mixed adenoneuroendocrine carcinomas and pure neuroendocrine carcinomas
Jiwon Koh, Soo Kyung Nam, Yoonjin Kwak, Gilhyang Kim, Ka‐Kyung Kim, Byung‐Chul Lee, Sang‐Hoon Ahn, Do Joong Park, Hyung‐Ho Kim, Kyoung Un Park, Woo Ho Kim, Hye Seung Lee
The Journal of Pathology.2021; 253(1): 94. CrossRef - Alterations of PTEN and SMAD4 methylation in diagnosis of breast cancer: implications of methyl II PCR assay
Menha Swellam, Entsar A. Saad, Shimaa Sabry, Adel Denewer, Camelia Abdel Malak, Amr Abouzid
Journal of Genetic Engineering and Biotechnology.2021; 19(1): 54. CrossRef - Molecular Characterization and Functional Analysis of Two Steroidogenic Genes TSPO and SMAD4 in Yellow Catfish
Fang Chen, Chong-Chao Zhong, Chang-Chun Song, Shu-Wei Chen, Yang He, Xiao-Ying Tan
International Journal of Molecular Sciences.2021; 22(9): 4505. CrossRef - SMAD7 and SMAD4 expression in colorectal cancer progression and therapy response
Jovana Rosic, Sandra Dragicevic, Marko Miladinov, Jovana Despotovic, Aleksandar Bogdanovic, Zoran Krivokapic, Aleksandra Nikolic
Experimental and Molecular Pathology.2021; 123: 104714. CrossRef - Actionable Potentials of Less Frequently Mutated Genes in Colorectal Cancer and Their Roles in Precision Medicine
Ryia Illani Mohd Yunos, Nurul Syakima Ab Mutalib, Francis Yew Fu Tieng, Nadiah Abu, Rahman Jamal
Biomolecules.2020; 10(3): 476. CrossRef
- Association between the expression of epithelial–mesenchymal transition (EMT)-related markers and oncologic outcomes of colorectal cancer
- CpG Island Methylation in Sessile Serrated Adenoma/Polyp of the Colorectum: Implications for Differential Diagnosis of Molecularly High-Risk Lesions among Non-dysplastic Sessile Serrated Adenomas/Polyps
- Ji Ae Lee, Hye Eun Park, Seung-Yeon Yoo, Seorin Jeong, Nam-Yun Cho, Gyeong Hoon Kang, Jung Ho Kim
- J Pathol Transl Med. 2019;53(4):225-235. Published online March 19, 2019
- DOI: https://doi.org/10.4132/jptm.2019.03.12
- 8,257 View
- 242 Download
- 7 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
Although colorectal sessile serrated adenomas/polyps (SSA/Ps) with morphologic dysplasia are regarded as definite high-risk premalignant lesions, no reliable grading or risk-stratifying system exists for non-dysplastic SSA/Ps. The accumulation of CpG island methylation is a molecular hallmark of progression of SSA/Ps. Thus, we decided to classify non-dysplastic SSA/Ps into risk subgroups based on the extent of CpG island methylation.
Methods
The CpG island methylator phenotype (CIMP) status of 132 non-dysplastic SSA/Ps was determined using eight CIMP-specific promoter markers. SSA/Ps with CIMP-high and/or MLH1 promoter methylation were regarded as a high-risk subgroup.
Results
Based on the CIMP analysis results, methylation frequency of each CIMP marker suggested a sequential pattern of CpG island methylation during progression of SSA/P, indicating MLH1 as a late-methylated marker. Among the 132 non-dysplastic SSA/Ps, 34 (26%) were determined to be high-risk lesions (33 CIMP-high and 8 MLH1-methylated cases; seven cases overlapped). All 34 high-risk SSA/Ps were located exclusively in the proximal colon (100%, p = .001) and were significantly associated with older age (≥ 50 years, 100%; p = .003) and a larger histologically measured lesion size (> 5 mm, 100%; p = .004). In addition, the high-risk SSA/Ps were characterized by a relatively higher number of typical base-dilated serrated crypts.
Conclusions
Both CIMP-high and MLH1 methylation are late-step molecular events during progression of SSA/Ps and rarely occur in SSA/Ps of young patients. Comprehensive consideration of age (≥ 50), location (proximal colon), and histologic size (> 5 mm) may be important for the prediction of high-risk lesions among non-dysplastic SSA/Ps. -
Citations
Citations to this article as recorded by- MLH1 Methylation Status and Microsatellite Instability in Patients with Colorectal Cancer
Manuel Alejandro Rico-Méndez, Miguel Angel Trujillo-Rojas, María de la Luz Ayala-Madrigal, Jesús Arturo Hernández-Sandoval, Anahí González-Mercado, Melva Gutiérrez-Angulo, José Geovanni Romero-Quintana, Jesús Alonso Valenzuela-Pérez, Ruth Ramírez-Ramírez,
Genes.2025; 16(2): 182. CrossRef - Immune microenvironmental heterogeneity according to tumor DNA methylation phenotypes in microsatellite instability-high colorectal cancers
Jung Ho Kim, Jiyun Hong, Ji Ae Lee, Minsun Jung, Eunwoo Choi, Nam-Yun Cho, Gyeong Hoon Kang, Sangwoo Kim
Cancer Immunology, Immunotherapy.2024;[Epub] CrossRef - How to "pick up" colorectal serrated lesions and polyps in daily histopathology practice: From terminologies to diagnostic pitfalls
Thai H Tran, Vinh H Nguyen, Diem TN Vo
World Journal of Clinical Oncology.2024; 15(9): 1157. CrossRef - Serrated Colorectal Lesions: An Up-to-Date Review from Histological Pattern to Molecular Pathogenesis
Martino Mezzapesa, Giuseppe Losurdo, Francesca Celiberto, Salvatore Rizzi, Antonio d’Amati, Domenico Piscitelli, Enzo Ierardi, Alfredo Di Leo
International Journal of Molecular Sciences.2022; 23(8): 4461. CrossRef - NTRK oncogenic fusions are exclusively associated with the serrated neoplasia pathway in the colorectum and begin to occur in sessile serrated lesions
Jung Ho Kim, Jeong Hoon Hong, Yoon‐La Choi, Ji Ae Lee, Mi‐kyoung Seo, Mi‐Sook Lee, Sung Bin An, Min Jung Sung, Nam‐Yun Cho, Sung‐Su Kim, Young Kee Shin, Sangwoo Kim, Gyeong Hoon Kang
The Journal of Pathology.2021; 255(4): 399. CrossRef - Evolving pathologic concepts of serrated lesions of the colorectum
Jung Ho Kim, Gyeong Hoon Kang
Journal of Pathology and Translational Medicine.2020; 54(4): 276. CrossRef
- MLH1 Methylation Status and Microsatellite Instability in Patients with Colorectal Cancer
- Prognostic Impact of Fusobacterium nucleatum Depends on Combined Tumor Location and Microsatellite Instability Status in Stage II/III Colorectal Cancers Treated with Adjuvant Chemotherapy
- Hyeon Jeong Oh, Jung Ho Kim, Jeong Mo Bae, Hyun Jung Kim, Nam-Yun Cho, Gyeong Hoon Kang
- J Pathol Transl Med. 2019;53(1):40-49. Published online December 26, 2018
- DOI: https://doi.org/10.4132/jptm.2018.11.29
- 17,586 View
- 249 Download
- 45 Web of Science
- 47 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
This study aimed to investigate the prognostic impact of intratumoral Fusobacterium nucleatum in colorectal cancer (CRC) treated with adjuvant chemotherapy.
Methods
F. nucleatumDNA was quantitatively measured in a total of 593 CRC tissues retrospectively collectedfrom surgically resected specimens of stage III or high-risk stage II CRC patients who had receivedcurative surgery and subsequent oxaliplatin-based adjuvant chemotherapy (either FOLFOXor CAPOX). Each case was classified into one of the three categories: F. nucleatum–high, –low, or –negative.
Results
No significant differences in survival were observed between the F.nucleatum–high and –low/negative groups in the 593 CRCs (p = .671). Subgroup analyses accordingto tumor location demonstrated that disease-free survival was significantly better in F.nucleatum–high than in –low/negative patients with non-sigmoid colon cancer (including cecal,ascending, transverse, and descending colon cancers; n = 219; log-rank p = .026). In multivariateanalysis, F. nucleatum was determined to be an independent prognostic factor in non-sigmoidcolon cancers (hazard ratio, 0.42; 95% confidence interval, 0.18 to 0.97; p = .043). Furthermore,the favorable prognostic effect of F. nucleatum–high was observed only in a non-microsatellite instability-high (non-MSI-high) subset of non-sigmoid colon cancers (log-rank p = 0.014), but not ina MSI-high subset (log-rank p = 0.844), suggesting that the combined status of tumor locationand MSI may be a critical factor for different prognostic impacts of F. nucleatum in CRCs treatedwith adjuvant chemotherapy.
Conclusions
Intratumoral F. nucleatum load is a potential prognosticfactor in a non-MSI-high/non-sigmoid/non-rectal cancer subset of stage II/III CRCs treatedwith oxaliplatin-based adjuvant chemotherapy. -
Citations
Citations to this article as recorded by- Unraveling the Role of Fusobacterium nucleatum in Colorectal Cancer: Molecular Mechanisms and Pathogenic Insights
Linda Galasso, Fabrizio Termite, Irene Mignini, Giorgio Esposto, Raffaele Borriello, Federica Vitale, Alberto Nicoletti, Mattia Paratore, Maria Elena Ainora, Antonio Gasbarrini, Maria Assunta Zocco
Cancers.2025; 17(3): 368. CrossRef - Emerging roles of intratumoral microbiota: a key to novel cancer therapies
Pengzhong Fang, Jing Yang, Huiyun Zhang, Diankui Shuai, Min Li, Lin Chen, Liping Liu
Frontiers in Oncology.2025;[Epub] CrossRef - Association of Fusobacterium nucleatum with colorectal cancer molecular subtypes and its outcome: a systematic review
Luana Greco, Federica Rubbino, Clarissa Ferrari, Michela Cameletti, Fabio Grizzi, Fabrizio Bonelli, Alberto Malesci, Massimiliano Mazzone, Luigi Ricciardiello, Luigi Laghi
Gut Microbiome.2025;[Epub] CrossRef - Composition of the colon microbiota in the individuals with inflammatory bowel disease and colon cancer
Ceren Acar, Sibel Kucukyildirim Celik, H. Ozgur Ozdemirel, Beril Erdem Tuncdemir, Saadet Alan, Hatice Mergen
Folia Microbiologica.2024; 69(2): 333. CrossRef - Intratumoral microorganisms in tumors of the digestive system
Mengjuan Xuan, Xinyu Gu, Yingru Liu, Li Yang, Yi Li, Di Huang, Juan Li, Chen Xue
Cell Communication and Signaling.2024;[Epub] CrossRef - Prognostic impact of oral microbiome on survival of malignancies: a systematic review and meta-analysis
Shuluan Li, Tianyu Wang, Ya Ren, Zhou Liu, Jidong Gao, Zhi Guo
Systematic Reviews.2024;[Epub] CrossRef - Exploring the Potential of Humoral Immune Response to Commensal Bifidobacterium as a Biomarker for Human Health, including Both Malignant and Non-Malignant Diseases: A Perspective on Detection Strategies and Future Directions
Kyogo Itoh, Satoko Matsueda
Biomedicines.2024; 12(4): 803. CrossRef - Unveiling intratumoral microbiota: An emerging force for colorectal cancer diagnosis and therapy
Jinjing Zhang, Penghui Wang, Jiafeng Wang, Xiaojie Wei, Mengchuan Wang
Pharmacological Research.2024; 203: 107185. CrossRef - Spatial transcriptomic analysis reveals local effects of intratumoral fusobacterial infection on DNA damage and immune signaling in rectal cancer
William P. Duggan, Batuhan Kisakol, Ina Woods, Mohammedreza Azimi, Heiko Dussmann, Joanna Fay, Tony O’Grady, Barry Maguire, Ian S. Reynolds, Manuela Salvucci, Daniel J. Slade, Deborah A. McNamara, John P. Burke, Jochen H.M. Prehn
Gut Microbes.2024;[Epub] CrossRef - Gut microbiota characteristics of colorectal cancer patients in Hubei, China, and differences with cohorts from other Chinese regions
Jianguo Shi, Hexiao Shen, Hui Huang, Lifang Zhan, Wei Chen, Zhuohui Zhou, Yongling Lv, Kai Xiong, Zhiwei Jiang, Qiyi Chen, Lei Liu
Frontiers in Microbiology.2024;[Epub] CrossRef - The role of Fusobacterium nucleatum in cancer and its implications for clinical applications
Wanyi Luo, Juxi Han, Xian Peng, Xuedong Zhou, Tao Gong, Xin Zheng
Molecular Oral Microbiology.2024; 39(6): 417. CrossRef - Gut Microbiome and colorectal cancer: discovery of bacterial changes with metagenomics application in Turkısh population
Yakup Ulger, Anıl Delik, Hikmet Akkız
Genes & Genomics.2024; 46(9): 1059. CrossRef - Intratumoral Microbiota: Metabolic Influences and Biomarker Potential in Gastrointestinal Cancer
Xueyuan Bi, Jihan Wang, Cuicui Liu
Biomolecules.2024; 14(8): 917. CrossRef - Intratumoral Microbiota: Insights from Anatomical, Molecular, and Clinical Perspectives
Claudia Lombardo, Rosanna Fazio, Marta Sinagra, Giuseppe Gattuso, Federica Longo, Cinzia Lombardo, Mario Salmeri, Guido Nicola Zanghì, Carla Agata Erika Loreto
Journal of Personalized Medicine.2024; 14(11): 1083. CrossRef - Exploring the role of Fusobacterium nucleatum in colorectal cancer: implications for tumor proliferation and chemoresistance
Leila Dadgar-Zankbar, Zahra Elahi, Aref Shariati, Azad Khaledi, Shabnam Razavi, Amin Khoshbayan
Cell Communication and Signaling.2024;[Epub] CrossRef - Fusobacterium nucleatum Abundance is Associated with Cachexia in Colorectal Cancer Patients: The ColoCare Study
Mmadili N. Ilozumba, Tengda Lin, Sheetal Hardikar, Doratha A. Byrd, June L. Round, W. Zac Stephens, Andreana N. Holowatyj, Christy A. Warby, Victoria Damerell, Christopher I. Li, Jane C. Figueiredo, Adetunji T. Toriola, David Shibata, Gary C. Fillmore, Ba
Cancer Medicine.2024;[Epub] CrossRef - Intratumoral microbiota: roles in cancer initiation, development and therapeutic efficacy
Li Yang, Aitian Li, Ying Wang, Yi Zhang
Signal Transduction and Targeted Therapy.2023;[Epub] CrossRef - Increased Fusobacterium tumoural abundance affects immunogenicity in mucinous colorectal cancer and may be associated with improved clinical outcome
William P. Duggan, Manuela Salvucci, Batuhan Kisakol, Andreas U. Lindner, Ian S. Reynolds, Heiko Dussmann, Joanna Fay, Tony O’Grady, Daniel B. Longley, Fiona Ginty, Elizabeth Mc Donough, Daniel J. Slade, John P. Burke, Jochen H. M. Prehn
Journal of Molecular Medicine.2023; 101(7): 829. CrossRef -
Fusobacterium nucleatum Load Correlates with KRAS Mutation and Sessile Serrated Pathogenesis in Colorectal Adenocarcinoma
Koki Takeda, Minoru Koi, Yoshiki Okita, Sija Sajibu, Temitope O. Keku, John M. Carethers
Cancer Research Communications.2023; 3(9): 1940. CrossRef - La asociación entre Fusobacterium nucleatum y el cáncer colorrectal: una revisión sistemática y metaanálisis
Paola Villar-Ortega, Manuela Expósito-Ruiz, Miguel Gutiérrez-Soto, Miguel Ruiz-Cabello Jiménez, José María Navarro-Marí, José Gutiérrez-Fernández
Enfermedades Infecciosas y Microbiología Clínica.2022; 40(5): 224. CrossRef - The association between Fusobacterium nucleatum and cancer colorectal: A systematic review and meta-analysis
Paola Villar-Ortega, Manuela Expósito-Ruiz, Miguel Gutiérrez-Soto, Miguel Ruiz-Cabello Jiménez, José María Navarro-Marí, José Gutiérrez-Fernández
Enfermedades infecciosas y microbiologia clinica (English ed.).2022; 40(5): 224. CrossRef - Suppression of Berberine and Probiotics (in vitro and in vivo) on the Growth of Colon Cancer With Modulation of Gut Microbiota and Butyrate Production
Chao Huang, Ying Sun, Sheng-rong Liao, Zhao-xin Chen, Han-feng Lin, Wei-zeng Shen
Frontiers in Microbiology.2022;[Epub] CrossRef - Prognostic and clinicopathological significance of Fusobacterium nucleatum in colorectal cancer: a systemic review and meta-analysis
Younghoon Kim, Nam Yun Cho, Gyeong Hoon Kang
Journal of Pathology and Translational Medicine.2022; 56(3): 144. CrossRef - Iron accelerates Fusobacterium nucleatum–induced CCL8 expression in macrophages and is associated with colorectal cancer progression
Taishi Yamane, Yohei Kanamori, Hiroshi Sawayama, Hiromu Yano, Akihiro Nita, Yudai Ohta, Hironori Hinokuma, Ayato Maeda, Akiko Iwai, Takashi Matsumoto, Mayuko Shimoda, Mayumi Niimura, Shingo Usuki, Noriko Yasuda-Yoshihara, Masato Niwa, Yoshifumi Baba, Taka
JCI Insight.2022;[Epub] CrossRef - Clinicopathological differences of high Fusobacterium nucleatum levels in colorectal cancer: A review and meta-analysis
Yi Wang, Yuting Wen, Jiayin Wang, Xin Lai, Ying Xu, Xuanping Zhang, Xiaoyan Zhu, Chenglin Ruan, Yao Huang
Frontiers in Microbiology.2022;[Epub] CrossRef - Clinical Significance of Fusobacterium nucleatum and Microsatellite Instability in Evaluating Colorectal Cancer Prognosis
Yanxuan Xie, Xiaoyang Jiao, Mi Zeng, Zhiqiang Fan, Xin Li, Yumeng Yuan, Qiaoxin Zhang, Yong Xia
Cancer Management and Research.2022; Volume 14: 3021. CrossRef - Influence of the Microbiome Metagenomics and Epigenomics on Gastric Cancer
Precious Mathebela, Botle Precious Damane, Thanyani Victor Mulaudzi, Zilungile Lynette Mkhize-Khwitshana, Guy Roger Gaudji, Zodwa Dlamini
International Journal of Molecular Sciences.2022; 23(22): 13750. CrossRef - Circulating IgA Antibodies Against Fusobacterium nucleatum Amyloid Adhesin FadA are a Potential Biomarker for Colorectal Neoplasia
Jung Eun Baik, Li Li, Manish A. Shah, Daniel E. Freedberg, Zhezhen Jin, Timothy C. Wang, Yiping W. Han
Cancer Research Communications.2022; 2(11): 1497. CrossRef - Differential immune microenvironmental features of microsatellite-unstable colorectal cancers according to Fusobacterium nucleatum status
Ji Ae Lee, Seung-Yeon Yoo, Hyeon Jeong Oh, Seorin Jeong, Nam-Yun Cho, Gyeong Hoon Kang, Jung Ho Kim
Cancer Immunology, Immunotherapy.2021; 70(1): 47. CrossRef - Fusobacterium nucleatum and Clinicopathologic Features of Colorectal Cancer: Results From the ColoCare Study
Yannick Eisele, Patrick M. Mallea, Biljana Gigic, W. Zac Stephens, Christy A. Warby, Kate Buhrke, Tengda Lin, Juergen Boehm, Petra Schrotz-King, Sheetal Hardikar, Lyen C. Huang, T. Bartley Pickron, Courtney L. Scaife, Richard Viskochil, Torsten Koelsch, A
Clinical Colorectal Cancer.2021; 20(3): e165. CrossRef - Role of gut microbiota in epigenetic regulation of colorectal Cancer
Yinghui Zhao, Chuanxin Wang, Ajay Goel
Biochimica et Biophysica Acta (BBA) - Reviews on Cancer.2021; 1875(1): 188490. CrossRef - Fusobacterium nucleatum: caution with interpreting historical patient sample cohort
Kate L. F. Johnstone, Sinead Toomey, Stephen Madden, Brian D. P. O’Neill, Bryan T Hennessy
Journal of Pathology and Translational Medicine.2021; 55(6): 415. CrossRef -
Fusobacterium nucleatum colonization is associated with decreased survival of helicobacter pylori-positive gastric cancer patients
Yung-Yu Hsieh, Shui-Yi Tung, Hung-Yu Pan, Te-Sheng Chang, Kuo-Liang Wei, Wei-Ming Chen, Yi-Fang Deng, Chung-Kuang Lu, Yu-Hsuan Lai, Cheng-Shyong Wu, Chin Li
World Journal of Gastroenterology.2021; 27(42): 7311. CrossRef - Analysis of changes in microbiome compositions related to the prognosis of colorectal cancer patients based on tissue-derived 16S rRNA sequences
Sukjung Choi, Jongsuk Chung, Mi-La Cho, Donghyun Park, Sun Shim Choi
Journal of Translational Medicine.2021;[Epub] CrossRef - Gastrointestinal tumors and infectious agents: A wide field to explore
Miriam López-Gómez, Belén García de Santiago, Pedro-David Delgado-López, Eduardo Malmierca, Jesús González-Olmedo, César Gómez-Raposo, Carmen Sandoval, Pilar Ruiz-Seco, Nora Escribano, Jorge Francisco Gómez-Cerezo, Enrique Casado
World Journal of Meta-Analysis.2021; 9(6): 505. CrossRef - Gut Microbiota Profiles in Early- and Late-Onset Colorectal Cancer: A Potential Diagnostic Biomarker in the Future
Murdani Abdullah, Ninik Sukartini, Saskia Aziza Nursyirwan, Rabbinu Rangga Pribadi, Hasan Maulahela, Amanda Pitarini Utari, Virly Nanda Muzellina, Agustinus Wiraatmadja, Kaka Renaldi
Digestion.2021; 102(6): 823. CrossRef - The effect of periodontal bacteria infection on incidence and prognosis of cancer
Li Xiao, Qianyu Zhang, Yanshuang Peng, Daqing Wang, Ying Liu
Medicine.2020; 99(15): e19698. CrossRef - The impact of the gut microbiota on prognosis after surgery for colorectal cancer – a systematic review and meta‐analysis
Emilie Palmgren Colov, Thea Helene Degett, Hans Raskov, Ismail Gögenur
APMIS.2020; 128(2): 162. CrossRef - Can the microbiota predict response to systemic cancer therapy, surgical outcomes, and survival? The answer is in the gut
Khalid El Bairi, Rachid Jabi, Dario Trapani, Hanae Boutallaka, Bouchra Ouled Amar Bencheikh, Mohammed Bouziane, Mariam Amrani, Said Afqir, Adil Maleb
Expert Review of Clinical Pharmacology.2020; 13(4): 403. CrossRef - Predictive Values of Colon Microbiota in the Treatment Response to Colorectal Cancer
Jorge Galan-Ros, Verónica Ramos-Arenas, Pablo Conesa-Zamora
Pharmacogenomics.2020; 21(14): 1045. CrossRef - The gut microbiome and potential implications for early-onset colorectal cancer
Reetu Mukherji, Benjamin A Weinberg
Colorectal Cancer.2020;[Epub] CrossRef -
Fusobacterium nucleatum in the Colorectum and Its Association with Cancer Risk and Survival: A Systematic Review and Meta-analysis
Christian Gethings-Behncke, Helen G. Coleman, Haydee W.T. Jordao, Daniel B. Longley, Nyree Crawford, Liam J. Murray, Andrew T. Kunzmann
Cancer Epidemiology, Biomarkers & Prevention.2020; 29(3): 539. CrossRef - CpG Island Methylation in Sessile Serrated Adenoma/Polyp of the Colorectum: Implications for Differential Diagnosis of Molecularly High-Risk Lesions among Non-dysplastic Sessile Serrated Adenomas/Polyps
Ji Ae Lee, Hye Eun Park, Seung-Yeon Yoo, Seorin Jeong, Nam-Yun Cho, Gyeong Hoon Kang, Jung Ho Kim
Journal of Pathology and Translational Medicine.2019; 53(4): 225. CrossRef - Fusobacterium nucleatum tumor DNA levels are associated with survival in colorectal cancer patients
Andrew T. Kunzmann, Marcela Alcântara Proença, Haydee WT Jordao, Katerina Jiraskova, Michaela Schneiderova, Miroslav Levy, Václav Liska, Tomas Buchler, Ludmila Vodickova, Veronika Vymetalkova, Ana Elizabete Silva, Pavel Vodicka, David J. Hughes
European Journal of Clinical Microbiology & Infectious Diseases.2019; 38(10): 1891. CrossRef - Gut Microbiome: A Promising Biomarker for Immunotherapy in Colorectal Cancer
Sally Temraz, Farah Nassar, Rihab Nasr, Maya Charafeddine, Deborah Mukherji, Ali Shamseddine
International Journal of Molecular Sciences.2019; 20(17): 4155. CrossRef - The Four Horsemen in Colon Cancer
Marco Antonio Hernández-Luna, Sergio López-Briones, Rosendo Luria-Pérez
Journal of Oncology.2019; 2019: 1. CrossRef - The role of Fusobacterium nucleatum in colorectal cancer: from carcinogenesis to clinical management
Chun‐Hui Sun, Bin‐Bin Li, Bo Wang, Jing Zhao, Xiao‐Ying Zhang, Ting‐Ting Li, Wen‐Bing Li, Di Tang, Miao‐Juan Qiu, Xin‐Cheng Wang, Cheng‐Ming Zhu, Zhi‐Rong Qian
Chronic Diseases and Translational Medicine.2019; 5(3): 178. CrossRef
- Unraveling the Role of Fusobacterium nucleatum in Colorectal Cancer: Molecular Mechanisms and Pathogenic Insights
- Prognostic Significance of EPHB2 Expression in Colorectal Cancer Progression
- Bo Gun Jang, Hye Sung Kim, Weon Young Chang, Jeong Mo Bae, Gyeong Hoon Kang
- J Pathol Transl Med. 2018;52(5):298-306. Published online July 18, 2018
- DOI: https://doi.org/10.4132/jptm.2018.06.29
- 7,526 View
- 155 Download
- 16 Web of Science
- 12 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
A receptor tyrosine kinase for ephrin ligands, EPHB2, is expressed in normal colorectal tissues and colorectal cancers (CRCs). The aim of this study was to investigate EPHB2 expression over CRC progression and determine its prognostic significance in CRC.
Methods
To measure EPHB2 mRNA and protein expression, real-time polymerase chain reaction and immunohistochemistry were performed in 32 fresh-frozen and 567 formalin-fixed paraffin-embedded CRC samples, respectively. We further investigated clinicopathological features and overall and recurrence-free survival according to EPHB2 protein expression.
Results
The EPHB2 level was upregulated in CRC samples compared to non-cancerous tissue in most samples and showed a strong positive correlation with AXIN2. Notably, CD44 had a positive association with both mRNA and protein levels of EPHB2. Immunohistochemical analysis revealed no difference in EPHB2 expression between adenoma and carcinoma areas. Although EPHB2 expression was slightly lower in invasive fronts compared to surface area (p < .05), there was no difference between superficial and metastatic areas. EPHB2 positivity was associated with lymphatic (p < .001) and venous (p = .001) invasion, TNM stage (p < .001), and microsatellite instability (p = .036). Kaplan–Meier analysis demonstrated that CRC patients with EPHB2 positivity showed better clinical outcomes in both overall (p = .049) and recurrence-free survival (p = .015). However, multivariate analysis failed to show that EPHB2 is an independent prognostic marker in CRCs (hazard ratio, 0.692; p = .692).
Conclusions
Our results suggest that EPHB2 is overexpressed in a subset of CRCs and is a significant prognostic marker. -
Citations
Citations to this article as recorded by- Potential role of the Eph/ephrin system in colorectal cancer: emerging druggable molecular targets
João Figueira Scarini, Moisés Willian Aparecido Gonçalves, Reydson Alcides de Lima-Souza, Luccas Lavareze, Talita de Carvalho Kimura, Ching-Chu Yang, Albina Altemani, Fernanda Viviane Mariano, Heloisa Prado Soares, Gary Chris Fillmore, Erika Said Abu Egal
Frontiers in Oncology.2024;[Epub] CrossRef - Comprehensive pan-cancer analysis reveals EPHB2 is a novel predictive biomarker for prognosis and immunotherapy response
Shengshan Xu, Youbin Zheng, Min Ye, Tao Shen, Dongxi Zhang, Zumei Li, Zhuming Lu
BMC Cancer.2024;[Epub] CrossRef - Therapeutic effects of ephrin B receptor 2 inhibitors screened by molecular docking on cutaneous squamous cell carcinoma
Yan Li, Xuanfen Zhang
Journal of Dermatological Treatment.2022; 33(1): 373. CrossRef - The EPH/Ephrin System in Colorectal Cancer
Stavros P. Papadakos, Leonidas Petrogiannopoulos, Alexandros Pergaris, Stamatios Theocharis
International Journal of Molecular Sciences.2022; 23(5): 2761. CrossRef - Prognostic Significance of Autophagy-Relevant Gene Markers in Colorectal Cancer
Qinglian He, Ziqi Li, Jinbao Yin, Yuling Li, Yuting Yin, Xue Lei, Wei Zhu
Frontiers in Oncology.2021;[Epub] CrossRef - Genetic Predisposition to Numerous Large Ulcerating Basal Cell Carcinomas and Response to Immune Therapy
Bahar Dasgeb, Leila Youssefian, Amir Hossein Saeidian, Jun Kang, Wenyin Shi, Elizabeth Shoenberg, Adam Ertel, Paolo Fortina, Hassan Vahidnezhad, Jouni Uitto
International Journal of Dermatology and Venereology.2021; 4(2): 70. CrossRef - Delineation of colorectal cancer ligand-receptor interactions and their roles in the tumor microenvironment and prognosis
Hexin Lin, Lu Xia, Jiabian Lian, Yinan Chen, Yiyi Zhang, Zhicheng Zhuang, HuaJun Cai, Jun You, Guoxian Guan
Journal of Translational Medicine.2021;[Epub] CrossRef - Downregulation of hsa-microRNA-204-5p and identification of its potential regulatory network in non-small cell lung cancer: RT-qPCR, bioinformatic- and meta-analyses
Chang-Yu Liang, Zu-Yun Li, Ting-Qing Gan, Ye-Ying Fang, Bin-Liang Gan, Wen-Jie Chen, Yi-Wu Dang, Ke Shi, Zhen-Bo Feng, Gang Chen
Respiratory Research.2020;[Epub] CrossRef - The role of Eph receptors in cancer and how to target them: novel approaches in cancer treatment
Oscar J Buckens, Btissame El Hassouni, Elisa Giovannetti, Godefridus J Peters
Expert Opinion on Investigational Drugs.2020; 29(6): 567. CrossRef - Expression Profile and Prognostic Significance of EPHB3 in Colorectal Cancer
Bo Jang, Hye Kim, Jeong Bae, Woo Kim, Chang Hyun, Gyeong Kang
Biomolecules.2020; 10(4): 602. CrossRef Gene Expression Signature to Predict Prognosis and Adjuvant Chemosensitivity of Colorectal Cancer Patients
Jianxia Li, Jianwei Zhang, Huabin Hu, Yue Cai, Jiayu Ling, Zehua Wu, Yanhong Deng
Cancer Management and Research.2020; Volume 12: 3301. CrossRef- SMOC2, an intestinal stem cell marker, is an independent prognostic marker associated with better survival in colorectal cancers
Bo Gun Jang, Hye Sung Kim, Jeong Mo Bae, Woo Ho Kim, Heung Up Kim, Gyeong Hoon Kang
Scientific Reports.2020;[Epub] CrossRef
- Potential role of the Eph/ephrin system in colorectal cancer: emerging druggable molecular targets
- Utility of BRAF VE1 Immunohistochemistry as a Screening Tool for Colorectal Cancer Harboring BRAF V600E Mutation
- Jeong-Hwa Kwon, Byung-Kwan Jeong, Yong Sik Yoon, Chang Sik Yu, Jihun Kim
- J Pathol Transl Med. 2018;52(3):157-163. Published online March 29, 2018
- DOI: https://doi.org/10.4132/jptm.2018.03.28
- 7,874 View
- 196 Download
- 11 Web of Science
- 7 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
BRAF mutation has been recognized as an important biomarker of colorectal cancer (CRC) for targeted therapy and prognosis prediction. However, sequencing for every CRC case is not cost-effective. An antibody specific for BRAF V600E mutant protein has been introduced, and we thus examined the utility of BRAF VE1 immunohistochemistry for evaluating BRAF mutations in CRC.
Methods
Fifty-one BRAF-mutated CRCs and 100 age and sexmatched BRAF wild-type CRCs between 2005 and 2015 were selected from the archives of Asan Medical Center. Tissue microarrays were constructed and stained with BRAF VE1 antibody.
Results
Forty-nine of the 51 BRAF-mutant CRCs (96.1%) showed more than moderate cytoplasmic staining, except for two weakly stained cases. Six of 100 BRAF wild-type cases also stained positive with BRAF VE1 antibody; four stained weakly and two stained moderately. Normal colonic crypts showed nonspecific weak staining, and a few CRC cases exhibited moderate nuclear reactivity (3 BRAF-mutant and 10 BRAF wild-type cases). BRAF-mutated CRC patients had higher pathologic stages and worse survival than BRAF wild-type patients.
Conclusions
BRAF VE1 immunohistochemistry showed high sensitivity and specificity, but occasional nonspecific staining in tumor cell nuclei and normal colonic crypts may limit their routine clinical use. Thus, BRAF VE1 immunohistochemistry may be a useful screening tool for BRAF V600E mutation in CRCs, provided that additional sequencing studies can be done to confirm the mutation in BRAF VE1 antibody-positive cases. -
Citations
Citations to this article as recorded by- Next-Generation Sequencing in Oncology—A Guiding Compass for Targeted Therapy and Emerging Applications
Laurenția Nicoleta Galeș, Mihai-Andrei Păun, Ioana Butnariu, Laurentiu Simion, Loredana Sabina Cornelia Manolescu, Oana Gabriela Trifănescu, Rodica Maricela Anghel
International Journal of Molecular Sciences.2025; 26(7): 3123. CrossRef - The evolving landscape of tissue‐agnostic therapies in precision oncology
Vivek Subbiah, Mohamed A. Gouda, Bettina Ryll, Howard A. Burris, Razelle Kurzrock
CA: A Cancer Journal for Clinicians.2024; 74(5): 433. CrossRef - Deciphering the Role of BRAFV600E Immunohistochemistry in Breast Lesions: A Comprehensive Review
Simran Khan, Arvind Bhake, Shakti Sagar
Cureus.2024;[Epub] CrossRef - Immunohistochemistry as a Surrogate Marker of Underlying Molecular Derangements in Sporadic Colorectal Carcinoma in Children – A Series of Three Cases
Priyanka Maity, Aniket Halder, Ranajoy Ghosh, Uttara Chatterjee, Shibsankar Barman, Ruchirendra Sarkar
Fetal and Pediatric Pathology.2022; 41(1): 98. CrossRef - Risk assessment and genetic counseling for Lynch syndrome – Practice resource of the National Society of Genetic Counselors and the Collaborative Group of the Americas on Inherited Gastrointestinal Cancer
Spring Holter, Michael J. Hall, Heather Hampel, Kory Jasperson, Sonia S. Kupfer, Joy Larsen Haidle, Maureen E. Mork, Selvi Palaniapppan, Leigha Senter, Elena M. Stoffel, Scott M. Weissman, Matthew B. Yurgelun
Journal of Genetic Counseling.2022; 31(3): 568. CrossRef - Current concepts in ameloblastoma-targeted therapies in B-raf proto-oncogene serine/threonine kinase V600E mutation: Systematic review
Rogelio González-González, Sandra López-Verdín, Jesús Lavalle-Carrasco, Nelly Molina-Frechero, Mario Isiordia-Espinoza, Ramón G Carreón-Burciaga, Ronell Bologna-Molina
World Journal of Clinical Oncology.2020; 11(1): 31. CrossRef - Genetic and histopathological analysis of a case of primary intraosseous carcinoma, NOS with features of both ameloblastic carcinoma and squamous cell carcinoma
Akane Yukimori, Maiko Tsuchiya, Akane Wada, Yasuyuki Michi, Kou Kayamori, Kei Sakamoto, Tohru Ikeda
World Journal of Surgical Oncology.2020;[Epub] CrossRef
- Next-Generation Sequencing in Oncology—A Guiding Compass for Targeted Therapy and Emerging Applications

 E-submission
E-submission



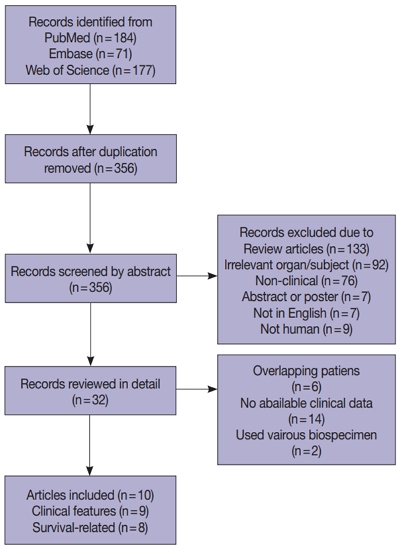





 First
First Prev
Prev



