Search
- Page Path
- HOME > Search
Review
- Cytologic hallmarks and differential diagnosis of papillary thyroid carcinoma subtypes
- Agnes Stephanie Harahap, Chan Kwon Jung
- J Pathol Transl Med. 2024;58(6):265-282. Published online November 7, 2024
- DOI: https://doi.org/10.4132/jptm.2024.10.11
- 4,481 View
- 405 Download
- 2 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF - Papillary thyroid carcinoma (PTC) is the most common thyroid malignancy, characterized by a range of subtypes that differ in their cytologic features, clinical behavior, and prognosis. Accurate cytologic evaluation of PTC using fine-needle aspiration is essential but can be challenging due to the morphologic diversity among subtypes. This review focuses on the distinct cytologic characteristics of various PTC subtypes, including the classic type, follicular variant, tall cell, columnar cell, hobnail, diffuse sclerosing, Warthin-like, solid/trabecular, and oncocytic PTCs. Each subtype demonstrates unique nuclear features, architectural patterns, and background elements essential for diagnosis and differentiation from other thyroid lesions. Recognizing these distinct cytologic patterns is essential for identifying aggressive subtypes like tall cell, hobnail, and columnar cell PTCs, which have a higher risk of recurrence, metastasis, and poorer clinical outcomes. Additionally, rare subtypes such as diffuse sclerosing and Warthin-like PTCs present unique cytologic profiles that must be carefully interpreted to avoid diagnostic errors. The review also highlights the cytologic indicators of lymph node metastasis and high-grade features, such as differentiated high-grade thyroid carcinoma. The integration of molecular testing can further refine subtype diagnosis by identifying specific genetic mutations. A thorough understanding of these subtype-specific cytologic features and molecular profiles is vital for accurate diagnosis, risk stratification, and personalized management of PTC patients. Future improvements in diagnostic techniques and standardization are needed to enhance cytologic evaluation and clinical decision-making in thyroid cancer.
-
Citations
Citations to this article as recorded by- Nuclear pseudoinclusion is associated with BRAFV600E mutation: Analysis of nuclear features in papillary thyroid carcinoma
Agnes Stephanie Harahap, Dina Khoirunnisa, Salinah, Maria Francisca Ham
Annals of Diagnostic Pathology.2025; 75: 152434. CrossRef - 2025 Korean Thyroid Association Clinical Management Guideline on Active Surveillance for Low-Risk Papillary Thyroid Carcinoma
Eun Kyung Lee, Min Joo Kim, Seung Heon Kang, Bon Seok Koo, Kyungsik Kim, Mijin Kim, Bo Hyun Kim, Ji-hoon Kim, Shin Je Moon, Kyorim Back, Young Shin Song, Jong-hyuk Ahn, Hwa Young Ahn, Ho-Ryun Won, Won Sang Yoo, Min Kyoung Lee, Jeongmin Lee, Ji Ye Lee, Kyo
International Journal of Thyroidology.2025; 18(1): 30. CrossRef - Structure-based molecular screening and dynamic simulation of phytocompounds targeting VEGFR-2: a novel therapeutic approach for papillary thyroid carcinoma
Shuai Wang, Lingqian Zhang, Wenjun Zhang, Xiong Zeng, Jie Mei, Weidong Xiao, Lijie Yang
Frontiers in Pharmacology.2025;[Epub] CrossRef - 2025 Korean Thyroid Association Clinical Management Guideline on Active Surveillance for Low-Risk Papillary Thyroid Carcinoma
Eun Kyung Lee, Min Joo Kim, Seung Heon Kang, Bon Seok Koo, Kyungsik Kim, Mijin Kim, Bo Hyun Kim, Ji-hoon Kim, Shinje Moon, Kyorim Back, Young Shin Song, Jong-hyuk Ahn, Hwa Young Ahn, Ho-Ryun Won, Won Sang Yoo, Min Kyoung Lee, Jeongmin Lee, Ji Ye Lee, Kyon
Endocrinology and Metabolism.2025; 40(3): 307. CrossRef
- Nuclear pseudoinclusion is associated with BRAFV600E mutation: Analysis of nuclear features in papillary thyroid carcinoma
Original Article
- The combination of CDX2 expression status and tumor-infiltrating lymphocyte density as a prognostic factor in adjuvant FOLFOX-treated patients with stage III colorectal cancers
- Ji-Ae Lee, Hye Eun Park, Hye-Yeong Jin, Lingyan Jin, Seung Yeon Yoo, Nam-Yun Cho, Jeong Mo Bae, Jung Ho Kim, Gyeong Hoon Kang
- J Pathol Transl Med. 2025;59(1):50-59. Published online October 24, 2024
- DOI: https://doi.org/10.4132/jptm.2024.09.26
- 1,880 View
- 264 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
Colorectal carcinomas (CRCs) with caudal-type homeobox 2 (CDX2) loss are recognized to pursue an aggressive behavior but tend to be accompanied by a high density of tumor-infiltrating lymphocytes (TILs). However, little is known about whether there is an interplay between CDX2 loss and TIL density in the survival of patients with CRC.
Methods
Stage III CRC tissues were assessed for CDX2 loss using immunohistochemistry and analyzed for their densities of CD8 TILs in both intraepithelial (iTILs) and stromal areas using a machine learning-based analytic method.
Results
CDX2 loss was significantly associated with a higher density of CD8 TILs in both intraepithelial and stromal areas. Both CDX2 loss and a high CD8 iTIL density were found to be prognostic parameters and showed hazard ratios of 2.314 (1.050–5.100) and 0.378 (0.175–0.817), respectively, for cancer-specific survival. A subset of CRCs with retained CDX2 expression and a high density of CD8 iTILs showed the best clinical outcome (hazard ratio of 0.138 [0.023–0.826]), whereas a subset with CDX2 loss and a high density of CD8 iTILs exhibited the worst clinical outcome (15.781 [3.939–63.230]).
Conclusions
Altogether, a high density of CD8 iTILs did not make a difference in the survival of patients with CRC with CDX2 loss. The combination of CDX2 expression and intraepithelial CD8 TIL density was an independent prognostic marker in adjuvant chemotherapy-treated patients with stage III CRC.
Case Study
- EWSR1 rearranged primary renal myoepithelial carcinoma: a diagnostic conundrum
- Nilay Nishith, Zachariah Chowdhury
- J Pathol Transl Med. 2023;57(5):284-288. Published online September 15, 2023
- DOI: https://doi.org/10.4132/jptm.2023.08.08
- 2,447 View
- 206 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF - Primary renal myoepithelial carcinoma is an exceedingly rare neoplasm with an aggressive phenotype and Ewing sarcoma breakpoint region 1 (EWSR1) rearrangement in a small fraction of cases. In addition to its rarity, the diagnosis can be challenging for the pathologist due to morphologic heterogeneity, particularly on the biopsy specimen. At times, immunohistochemistry may be indecisive; therefore, molecular studies should be undertaken for clinching the diagnosis. We aim to illustrate a case of primary myoepithelial carcinoma of the kidney with EWSR1-rearrangement in a 67-year-old male patient who presented with right supraclavicular mass, which was clinically diagnosed as carcinoma of an unknown primary. An elaborate immunohistochemical work-up aided by fluorescent in-situ hybridization allowed us to reach a conclusive diagnosis. This unusual case report advocates that one should be aware of the histological mimickers and begin with broad differential diagnoses alongside sporadic ones and then narrow them down with appropriate ancillary studies.
-
Citations
Citations to this article as recorded by- Primary Ewing Sarcoma of the Kidney
João Lobo, Huiying He, Raheel Ahmed, Bassel Zein-Sabatto, Thomas Winokur, Shi Wei, Shuko Harada, Jesse K. McKenney, Jonathan L. Myles, Jane K. Nguyen, Christopher G. Przybycin, Sean R. Williamson, Cristina Magi-Galluzzi, Reza Alaghehbandan
American Journal of Surgical Pathology.2025;[Epub] CrossRef
- Primary Ewing Sarcoma of the Kidney
Original Article
- Loss of aquaporin-1 expression is associated with worse clinical outcomes in clear cell renal cell carcinoma: an immunohistochemical study
- Seokhyeon Lee, Bohyun Kim, Minsun Jung, Kyung Chul Moon
- J Pathol Transl Med. 2023;57(4):232-237. Published online July 11, 2023
- DOI: https://doi.org/10.4132/jptm.2023.06.17
- 3,100 View
- 160 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Aquaporin (AQP) expression has been investigated in various malignant neoplasms, and the overexpression of AQP is related to poor prognosis in some malignancies. However, the expression of AQP protein in clear cell renal cell carcinoma (ccRCC) has not been extensively investigated by immunohistochemistry with large sample size.
Methods
We evaluated the AQP expression in 827 ccRCC with immunohistochemical staining in tissue microarray blocks and classified the cases into two categories, high and low expression.
Results
High expression of aquaporin-1 (AQP1) was found in 320 cases (38.7%), but aquaporin-3 was not expressed in ccRCC. High AQP1 expression was significantly related to younger age, low TNM stage, low World Health Organization/International Society of Urologic Pathology nuclear grade, and absence of distant metastasis. Furthermore, high AQP1 expression was also significantly associated with longer overall survival (OS; p<.001) and progression-specific survival (PFS; p<.001) and was an independent predictor of OS and PFS in ccRCC.
Conclusions
Our study revealed the prognostic significance of AQP1 protein expression in ccRCC. These findings could be applied to predict the prognosis of ccRCC. -
Citations
Citations to this article as recorded by- Construction and validation of renal cell carcinoma tumor cell differentiation-related prognostic classification (RCC-TCDC): an integrated bioinformatic analysis and clinical study
Yifan Liu, Keqin Dong, Yuntao Yao, Bingnan Lu, Lei Wang, Guo Ji, Haoyu Zhang, Zihui Zhao, Xinyue Yang, Runzhi Huang, Wang Zhou, Xiuwu Pan, Xingang Cui
Annals of Medicine.2025;[Epub] CrossRef - Serum Exosomal MiR-874 as a Potential Biomarker for Nonsmall Cell Lung Cancer Diagnosis and Prognosis
Amal F. Gharib, Saad S. Al-Shehri, Abdulraheem Almalki, Ayman Alhazmi, Mamdouh Allahyani, Ahmed Alghamdi, Amani A. Alrehaili, Maha M. Bakhuraysah, Althobaiti Naif Saad M., Weal H. Elsawy
Indian Journal of Medical and Paediatric Oncology.2024;[Epub] CrossRef
- Construction and validation of renal cell carcinoma tumor cell differentiation-related prognostic classification (RCC-TCDC): an integrated bioinformatic analysis and clinical study
Case Study
- Unusual biclonal IgA plasma cell myeloma with aberrant expression of high-risk immunophenotypes: first report of a new diagnostic and clinical challenge
- Carlos A. Monroig-Rivera, Clara N. Finch Cruz
- J Pathol Transl Med. 2023;57(2):132-137. Published online March 14, 2023
- DOI: https://doi.org/10.4132/jptm.2023.02.07
- 3,158 View
- 133 Download
-
 Abstract
Abstract
 PDF
PDF - IgA plasma cell myeloma (PCM) has been linked to molecular abnormalities that confer a higher risk for adverse patient outcomes. However, since IgA PCM only accounts for approximately 20% of all PCM, there are very few reports on high-risk IgA PCM. Moreover, no such reports are found on the more infrequent biclonal IgA PCM. Hence, we present a 65-year-old Puerto Rican female with acute abdominal pain, concomitant hypercalcemia, and acute renal failure. Protein electrophoresis with immunofixation found high IgA levels and detected a biclonal IgA gammopathy with kappa specificity. Histomorphologically, bone marrow showed numerous abnormal plasma cells (32%) replacing over 50% of the marrow stroma. Immunophenotyping analysis detected CD45-negative plasma cells aberrantly expressing CD33, CD43, OCT-2, and c-MYC. Chromosomal analysis revealed multiple abnormalities including the gain of chromosome 1q. Thus, we report on an unusual biclonal IgA PCM and the importance of timely diagnosing aggressive plasma cell neoplasms.
Original Article
- Prognostic and clinicopathological significance of Fusobacterium nucleatum in colorectal cancer: a systemic review and meta-analysis
- Younghoon Kim, Nam Yun Cho, Gyeong Hoon Kang
- J Pathol Transl Med. 2022;56(3):144-151. Published online May 15, 2022
- DOI: https://doi.org/10.4132/jptm.2022.03.13
- 5,807 View
- 135 Download
- 8 Web of Science
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
Fusobacterium nucleatum has been identified to promote tumor progression in colorectal cancer (CRC). However, association between F. nucleatum and prognostic or clinicopathological features has been diverse among studies, which could be affected by type of biospecimen (formalin-fixed paraffin-embedded or fresh frozen [FF]).
Methods
Articles were systemically reviewed for studies that included the correlation between F. nucleatum and prognosis or clinicopathological features in CRC.
Results
Ten articles, eight studies with survival-related features involving 3,199 patients and nine studies with clinical features involving 2,655 patients, were eligible for the meta-analysis. Overall survival, disease-free survival, and cancer-specific survival were all associated with worse prognosis in F. nucleatum–high patients (p<.05). In subgroup analysis, only studies with FF tissues retained prognostic significance with F. nucleatum. In meta-analysis of clinicopathological variables, F. nucleatum level was associated with location within colon, pT category, MLH1 hypermethylation, microsatellite instability status, and BRAF mutation regardless of type of biospecimen. However, lymph node metastasis and KRAS mutation was only associated with F. nucleatum level in FF-based studies.
Conclusions
In conclusion, type of biospecimen could affect the role of F. nucleatum as a biomarker associated with clinicopathological features and prognosis. -
Citations
Citations to this article as recorded by- Unraveling the Role of Fusobacterium nucleatum in Colorectal Cancer: Molecular Mechanisms and Pathogenic Insights
Linda Galasso, Fabrizio Termite, Irene Mignini, Giorgio Esposto, Raffaele Borriello, Federica Vitale, Alberto Nicoletti, Mattia Paratore, Maria Elena Ainora, Antonio Gasbarrini, Maria Assunta Zocco
Cancers.2025; 17(3): 368. CrossRef - Intratumoural pks Escherichia coli is associated with risk of metachronous colorectal cancer and adenoma development in people with Lynch syndrome
Yen Lin Chu, Peter Georgeson, Mark Clendenning, Khalid Mahmood, Romy Walker, Julia Como, Sharelle Joseland, Susan G. Preston, Toni Rice, Brigid M. Lynch, Roger L. Milne, Melissa C. Southey, Graham G. Giles, Amanda I. Phipps, John L. Hopper, Aung K. Win, C
eBioMedicine.2025; 114: 105661. CrossRef - Fusobacterium nucleatum Enrichment in Colorectal Tumor Tissue: Associations With Tumor Characteristics and Survival Outcomes
Amanda I. Phipps, Courtney M. Hill, Genevieve Lin, Rachel C. Malen, Adriana M. Reedy, Orsalem Kahsai, Hamza Ammar, Keith Curtis, Ningxin Ma, Timothy W. Randolph, Jing Ma, Shuji Ogino, Polly A. Newcomb, Meredith AJ. Hullar
Gastro Hep Advances.2025; 4(6): 100644. CrossRef - Enhancing fibroblast–epithelial cell communications: Serpine2 as a key molecule in Fusobacterium nucleatum–promoted colon cancer
Xueke Li, Simin Luo, Yifang Jiang, Qiong Ma, Fengming You, Qixuan Kuang, Xi Fu, Chuan Zheng
Frontiers in Immunology.2025;[Epub] CrossRef - Intratumoral presence of the genotoxic gut bacteria pks+ E. coli, Enterotoxigenic Bacteroides fragilis, and Fusobacterium nucleatum and their association with clinicopathological and molecular features of colorectal cancer
Jihoon E. Joo, Yen Lin Chu, Peter Georgeson, Romy Walker, Khalid Mahmood, Mark Clendenning, Aaron L. Meyers, Julia Como, Sharelle Joseland, Susan G. Preston, Natalie Diepenhorst, Julie Toner, Danielle J. Ingle, Norelle L. Sherry, Andrew Metz, Brigid M. Ly
British Journal of Cancer.2024; 130(5): 728. CrossRef - The role of Fusobacterium nucleatum in cancer and its implications for clinical applications
Wanyi Luo, Juxi Han, Xian Peng, Xuedong Zhou, Tao Gong, Xin Zheng
Molecular Oral Microbiology.2024; 39(6): 417. CrossRef -
Fusobacterium nucleatum Load Correlates with KRAS Mutation and Sessile Serrated Pathogenesis in Colorectal Adenocarcinoma
Koki Takeda, Minoru Koi, Yoshiki Okita, Sija Sajibu, Temitope O. Keku, John M. Carethers
Cancer Research Communications.2023; 3(9): 1940. CrossRef - Tumour Colonisation of Parvimonas micra Is Associated with Decreased Survival in Colorectal Cancer Patients
Thyra Löwenmark, Anna Löfgren-Burström, Carl Zingmark, Ingrid Ljuslinder, Michael Dahlberg, Sofia Edin, Richard Palmqvist
Cancers.2022; 14(23): 5937. CrossRef
- Unraveling the Role of Fusobacterium nucleatum in Colorectal Cancer: Molecular Mechanisms and Pathogenic Insights
Review
- Follicular lymphoma: updates for pathologists
- Mahsa Khanlari, Jennifer R. Chapman
- J Pathol Transl Med. 2022;56(1):1-15. Published online December 27, 2021
- DOI: https://doi.org/10.4132/jptm.2021.09.29
- 18,487 View
- 882 Download
- 17 Web of Science
- 16 Crossref
-
 Abstract
Abstract
 PDF
PDF - Follicular lymphoma (FL) is the most common indolent B-cell lymphoma and originates from germinal center B-cells (centrocytes and centroblasts) of the lymphoid follicle. Tumorigenesis is believed to initiate early in precursor B-cells in the bone marrow (BM) that acquire the t(14;18)(q32;q21). These cells later migrate to lymph nodes to continue their maturation through the germinal center reaction, at which time they acquire additional genetic and epigeneticabnormalities that promote lymphomagenesis. FLs are heterogeneous in terms of their clinicopathologic features. Most FLs are indolent and clinically characterized by peripheral lymphadenopathy with involvement of the spleen, BM, and peripheral blood in a substantial subset of patients, sometimes accompanied by constitutional symptoms and laboratory abnormalities. Diagnosis is established by the histopathologic identification of a B-cell proliferation usually distributed in an at least partially follicular pattern, typically, but not always, in a lymph node biopsy. The B-cell proliferation is biologically of germinal center cell origin, thus shows an expression of germinal center-associated antigens as detected by immunophenotyping. Although many cases of FLs are typical and histopathologic features are straightforward, the biologic and histopathologic variability of FL is wide, and an accurate diagnosis of FL over this disease spectrum requires knowledge of morphologic variants that can mimic other lymphomas, and rarely non-hematologic malignancies, clinically unique variants, and pitfalls in the interpretation of ancillary studies. The overall survival for most patients is prolonged, but relapses are frequent. The treatment landscape in FL now includes the application of immunotherapy and targeted therapy in addition to chemotherapy.
-
Citations
Citations to this article as recorded by- Relapsed/Refractory Follicular Lymphoma: Current Advances and Emerging Perspectives
Giulio Caridà, Enrica Antonia Martino, Antonella Bruzzese, Daniele Caracciolo, Caterina Labanca, Francesco Mendicino, Eugenio Lucia, Virginia Olivito, Teresa Rossi, Antonino Neri, Ernesto Vigna, Pierfrancesco Tassone, Pierosandro Tagliaferri, Fortunato Mo
European Journal of Haematology.2025; 114(5): 775. CrossRef - Frequency and Distribution of Lymphomas in Northwestern India: A Retrospective Analysis of 923 Cases Using the Latest World Health Organization Classification 5th Edition
Immanuel Paul Thayakaran, Biren Parikh
Indian Journal of Hematology and Blood Transfusion.2025;[Epub] CrossRef - IGH/IGK gene rearrangement in the diagnosis of B-cell non-Hodgkin lymphoma: experience from three centers
Ke Yang, Zhizhong Wang, Beibei Xin, Yunhang Li, Jiuzhou Zhao, Rui Sun, Weizhen Wang, Dongxu Chen, Chengzhi Zhao, Yongjun Guo, Jie Ma, Bing Wei
Annals of Hematology.2025;[Epub] CrossRef - Imaging Evaluation of Periarticular Soft Tissue Masses in the Appendicular Skeleton: A Pictorial Review
Francesco Pucciarelli, Maria Carla Faugno, Daniela Valanzuolo, Edoardo Massaro, Lorenzo Maria De Sanctis, Elisa Zaccaria, Marta Zerunian, Domenico De Santis, Michela Polici, Tiziano Polidori, Andrea Laghi, Damiano Caruso
Journal of Imaging.2025; 11(7): 217. CrossRef - Transformation of low-grade follicular lymphoma to a high-grade follicular lymphoma with the histopathological diagnosis from oral biopsy: a case report
Gabriela Silveira de Araujo, Leandro Dorigan de Macedo, Alfredo Ribeiro-Silva, Hilton Marcos Alves Ricz, Lara Maria Alencar Ramos Innocentini
Hematology, Transfusion and Cell Therapy.2024; 46: S380. CrossRef - The follicular lymphoma tumor microenvironment at single-cell and spatial resolution
Andrea J. Radtke, Mark Roschewski
Blood.2024; 143(12): 1069. CrossRef - Chronic pancreatitis for the clinician: complications and special forms of the disease. Interdisciplinary position paper of the Catalan Society of Digestology (SCD) and the Catalan Pancreatic Society (SCPanc)
Xavier MOLERO, Juan R. AYUSO, Joaquim BALSELLS, Jaume BOADAS, Juli BUSQUETS, Anna CASTERÀS, Mar CONCEPCIÓN, Míriam CUATRECASAS, Gloria FERNÀNDEZ ESPARRACH, Esther FORT, Francisco GARCIA BOROBIA, Àngels GINÈS, Lucas ILZARBE, Carme LORAS, Miquel MASACHS, Xa
Minerva Gastroenterology.2024;[Epub] CrossRef - Concurrent identification of follicular lymphoma and papillary thyroid carcinoma
Lama A. Alzelfawi, Norah ALhumaidan, Abrar H. Alageel, Buthaina J. Yahya, Saud D. Alrasheedi, Adel S. Alqahtani
International Journal of Surgery Case Reports.2024; 122: 110009. CrossRef - Impact of Primary Disease Site of Involvement by Early-Stage Follicular Lymphoma on Patient Outcomes
Olivia Davis, Carmen Lessani, Rana Kasht, Andrew Cohoon, Sami Ibrahimi, Adam Asch, Silas Day, Taha Al-Juhaishi
Clinical Lymphoma Myeloma and Leukemia.2024; 24(12): 837. CrossRef - Recent developments in CD19-targeted therapies for follicular lymphoma
Aditi Saha, Julio C. Chavez
Expert Opinion on Biological Therapy.2024; 24(10): 1049. CrossRef - Unraveling the complexity of follicular lymphoma: insights and innovations
Xijing Li
American Journal of Cancer Research.2024; 14(12): 5573. CrossRef - Clinical features and prognostic factors in 49 patients with follicular lymphoma at a single center: A retrospective analysis
Hao Wu, Hui-Cong Sun, Gui-Fang Ouyang
World Journal of Clinical Cases.2023; 11(14): 3176. CrossRef - A rare case of follicular lymphoma of the bladder
Matthew DeSanto, Robert Strait, Jared Zopp, Kevin Brown, Samuel Deem
Urology Case Reports.2023; 51: 102542. CrossRef - Analysis of immunophenotypic features in hyaline vascular type Castleman disease
Yu Chang, Yu Ma, Chen Chang, Wensheng Li
Diagnostic Pathology.2023;[Epub] CrossRef - Leg Edema Unveiled: The Uncommon Culprit of Follicular Lymphoma
Syed Muhammad IbnE Ali Jaffari, Samaha Nisar, Narjis Malik, Syed Muhammad Aun Ali Jaffari, Omar Nisar
Journal of Shalamar Medical & Dental College - JSHMDC.2023; 4(2): 125. CrossRef - A Review of the Totality of Evidence in the Development of ABP 798, A Rituximab Biosimilar
Patrick Cobb, Dietger Niederwieser, Stanley Cohen, Caroline Hamm, Gerd Burmester, Neungseon Seo, Sonya G Lehto, Vladimir Hanes
Immunotherapy.2022; 14(9): 727. CrossRef
- Relapsed/Refractory Follicular Lymphoma: Current Advances and Emerging Perspectives
Original Articles
- Prognostic significance of viable tumor size measurement in hepatocellular carcinomas after preoperative locoregional treatment
- Yoon Jung Hwang, Youngeun Lee, Hyunjin Park, Yangkyu Lee, Kyoungbun Lee, Haeryoung Kim
- J Pathol Transl Med. 2021;55(5):338-348. Published online September 2, 2021
- DOI: https://doi.org/10.4132/jptm.2021.07.26
- 4,507 View
- 113 Download
- 5 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
Preoperative locoregional treatment (LRT) for hepatocellular carcinoma (HCC) often induces intratumoral necrosis without affecting the overall tumor size, and residual viable tumor size (VTS) on imaging is an important clinical parameter for assessing post-treatment response. However, for surgical specimens, it is unclear whether the VTS would be more relevant to prognosis compared to total tumor size (TTS).
Methods
A total of 142 surgically resected solitary HCC cases were retrospectively reviewed. The TTS and VTS were assessed by applying the modified Response Evaluation Criteria in Solid Tumors method to the resected specimens, and correlated with the clinicopathological features and survival.
Results
As applying VTS, 13/142 cases (9.2%) were down-staged to ypT1a. Although the survival analysis results for overall survival according to TTS or VTS were similar, VTS was superior to predict disease-free survival (DFS; p = .023) compared to TTS (p = .08). In addition, multivariate analysis demonstrated VTS > 2 cm to be an independent predictive factor for decreased DFS (p = .001). In the subpopulation of patients with LRT (n = 54), DFS in HCCs with TTS or VTS > 2 cm were significantly shorter than those with TTS or VTS ≤ 2 cm (p = .047 and p = .001, respectively). Interestingly, HCCs with TTS > 2 cm but down-staged to VTS ≤ 2 cm after preoperative LRT had similar survival to those with TTS ≤ 2 cm.
Conclusions
Although the prognostic impact of tumor size was similar regardless of whether TTS or VTS was applied, reporting VTS may help to increase the number of candidates for surgery in HCC patients with preoperative LRT. -
Citations
Citations to this article as recorded by- PET-Assessed Metabolic Tumor Volume Across the Spectrum of Solid-Organ Malignancies: A Review of the Literature
Anusha Agarwal, Chase J. Wehrle, Sangeeta Satish, Paresh Mahajan, Suneel Kamath, Shlomo Koyfman, Wen Wee Ma, Maureen Linganna, Jamak Modaresi Esfeh, Charles Miller, David C. H. Kwon, Andrea Schlegel, Federico Aucejo
Biomedicines.2025; 13(1): 123. CrossRef - Measures for response assessment in HCC treatment
Fereshteh Yazdanpanah, Omar Al-Daoud, Moein Moradpour, Stephen Hunt
Hepatoma Research.2024;[Epub] CrossRef - Machine Learning for Dynamic Prognostication of Patients With Hepatocellular Carcinoma Using Time-Series Data: Survival Path Versus Dynamic-DeepHit HCC Model
Lujun Shen, Yiquan Jiang, Tao Zhang, Fei Cao, Liangru Ke, Chen Li, Gulijiayina Nuerhashi, Wang Li, Peihong Wu, Chaofeng Li, Qi Zeng, Weijun Fan
Cancer Informatics.2024;[Epub] CrossRef - Construction and validation of a novel signature based on epithelial-mesenchymal transition–related genes to predict prognosis and immunotherapy response in hepatocellular carcinoma by comprehensive analysis of the tumor microenvironment
Biao Gao, Yafei Wang, Shichun Lu
Functional & Integrative Genomics.2023;[Epub] CrossRef - Cellular senescence affects energy metabolism, immune infiltration and immunotherapeutic response in hepatocellular carcinoma
Biao Gao, Yafei Wang, Shichun Lu
Scientific Reports.2023;[Epub] CrossRef
- PET-Assessed Metabolic Tumor Volume Across the Spectrum of Solid-Organ Malignancies: A Review of the Literature
- Prognostic role of ALK-1 and h-TERT expression in glioblastoma multiforme: correlation with ALK gene alterations
- Dalia Elsers, Doaa F. Temerik, Alia M. Attia, A. Hadia, Marwa T. Hussien
- J Pathol Transl Med. 2021;55(3):212-224. Published online May 11, 2021
- DOI: https://doi.org/10.4132/jptm.2021.03.15
- 5,058 View
- 128 Download
- 5 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Anaplastic lymphoma kinase (ALK) is a receptor tyrosine kinase that is expressed in the developing central and peripheral nervous systems during embryogenesis. Human telomerase reverse transcriptase (h-TERT) protein resumption is the main process of preservation of telomeres that maintains DNA integrity. The present study aims to evaluate the prognostic role of ALK-1 and h-TERT protein expression and their correlation with ALK gene alterations in glioblastoma multiforme (GBM).
Methods
The current study is a retrospective study on a cohort of patients with GBM (n = 53) that attempted to detect ALK gene alterations using fluorescence in situ hybridization. ALK-1 and h-TERT proteins were evaluated using immunohistochemistry.
Results
Score 3 ALK-1 expression was significantly associated with male sex, tumor multiplicity, Ki labeling index (Ki LI), and type of therapeutic modality. Score 3 h-TERT expression exhibited a significant association with Ki LI. ALK gene amplifications (ALK-A) were significantly associated with increased Ki LI and therapeutic modalities. Score 3 ALK-1 protein expression, score 3 h-TERT protein expression, and ALK-A were associated with poor overall survival (OS) and progression-free survival (PFS). Multivariate analysis for OS revealed that ALK gene alterations were an independent prognostic factor for OS and PFS.
Conclusions
High protein expression of both ALK-1 and h-TERT, as well as ALK-A had a poor impact on the prognosis of GBM. Further studies are needed to establish the underlying mechanisms. -
Citations
Citations to this article as recorded by- TERT Gene Mutation in Gliomas Cross‐Linked With (NTRK, PDL1, ALK, IDH, P53, EGFR, HER2): A Integrative Review TERT Gene Mutation in Gliomas
Gunter Gerson Santos, Guilherme Nobre Nogueira, Iasmin Maria Rodrigues Saldanha, Ana Gabriela Ponte Farias, Cauan Miranda Mateus, Osvaldo Mariano Viana Neto, Maria Jânia Teixeira
Journal of Surgical Oncology.2025; 131(6): 1202. CrossRef - Mapping chromatin remodelling in glioblastoma identifies epigenetic regulation of key molecular pathways and novel druggable targets
Claire Vinel, James Boot, Weiwei Jin, Nicola Pomella, Alexandra Hadaway, Charles Mein, Nicolae Radu Zabet, Silvia Marino
BMC Biology.2025;[Epub] CrossRef - Association of human telomerase reverse transcriptase promoter mutation with unfavorable prognosis in glioma: A systematic review and meta-analysis
Rongxuan Hua, Qiuxuan Li, Han Gao, Boya Wang, Chengwei He, Ying Wang, Sitian Zhang, Lei Gao, Hongwei Shang, Wen Wang, Jingdong Xu
Journal of Research in Medical Sciences.2023;[Epub] CrossRef - Immunohistochemical surrogates for molecular alterations for the classification and grading of gliomas
Viharkumar Patel, Sanda Alexandrescu
Seminars in Diagnostic Pathology.2022; 39(1): 78. CrossRef - Meme Kanseri Hastalarında hTERT Gen Ekspresyonunun Klinikopatolojik Önemi
Ebubekir DİRİCAN, Burak KANKAYA, Zeynep TATAR
Sağlık Bilimlerinde Değer.2022; 12(1): 22. CrossRef - Prognostic and predictive markers in glioblastoma and ALK overexpression
Jang-Hee Kim
Journal of Pathology and Translational Medicine.2021; 55(3): 236. CrossRef
- TERT Gene Mutation in Gliomas Cross‐Linked With (NTRK, PDL1, ALK, IDH, P53, EGFR, HER2): A Integrative Review TERT Gene Mutation in Gliomas
- MicroRNA-552 expression in colorectal cancer and its clinicopathological significance
- Joon Im, Soo Kyung Nam, Hye Seung Lee
- J Pathol Transl Med. 2021;55(2):125-131. Published online February 19, 2021
- DOI: https://doi.org/10.4132/jptm.2021.01.17
- 4,530 View
- 124 Download
- 3 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
MicroRNA-552 (miR-552) has been reported to correlate with the development and progression of various cancers, including colorectal cancer (CRC). This study aimed to investigate miR-552 expression in cancer tissue samples compared to normal mucosal tissue and its role as a diagnostic or prognostic marker in CRC patients.
Methods
Normal mucosal tissues and primary cancer tissues from 80 surgically resected CRC specimens were used. Quantitative real-time polymerase chain reaction was performed for miR-552 and U6 small nuclear RNA to analyze miR-552 expression and its clinicopathological significance. Immunohistochemistry for p53 and phosphatase and tension homolog (PTEN) was performed to evaluate their association with miR-552 expression.
Results
miR-552 expression was significantly higher in primary cancer tissues compared to normal mucosal tissues (p<.001). The expression level of miR552 was inversely correlated with that of PTEN (p=.068) and p53 (p=.004). Survival analysis showed that high miR-552 expression was associated with worse prognosis but this was not statistically significant (p=.255). However, patients with CRC having high miR-552 expression and loss of PTEN expression had significantly worse prognosis than others (p=.029).
Conclusions
Our results suggest that high miR-552 expression might be a potential diagnostic biomarker for CRC, and its combined analysis with PTEN expression can possibly be used as a prognostic marker. -
Citations
Citations to this article as recorded by- MicroRNAs involved in colorectal cancer, a rapid mini-systematic review
Sogol Shirzad, Majid Eterafi, Zeinab Karimi, Mahdi Barazesh
BMC Cancer.2025;[Epub] CrossRef - Diagnostic and Therapeutic Potential of Selected microRNAs in Colorectal Cancer: A Literature Review
Grzegorz Sychowski, Hanna Romanowicz, Wojciech Ciesielski, Piotr Hogendorf, Adam Durczyński, Beata Smolarz
Cancers.2025; 17(13): 2135. CrossRef - Blood miRNAs miR-549a, miR-552, and miR-592 serve as potential disease-specific panels to diagnose colorectal cancer
Soroush Akbar, Samaneh Mashreghi, Mohammad Reza Kalani, Akram Valanik, Farzaneh Ahmadi, Mahdi Aalikhani, Zahra Bazi
Heliyon.2024; 10(7): e28492. CrossRef - Integration of TE Induces Cancer Specific Alternative Splicing Events
Woo Ryung Kim, Eun Gyung Park, Yun Ju Lee, Woo Hyeon Bae, Du Hyeong Lee, Heui-Soo Kim
International Journal of Molecular Sciences.2022; 23(18): 10918. CrossRef
- MicroRNAs involved in colorectal cancer, a rapid mini-systematic review
- The prognostic significance of p16 expression pattern in diffuse gliomas
- Jin Woo Park, Jeongwan Kang, Ka Young Lim, Hyunhee Kim, Seong-Ik Kim, Jae Kyung Won, Chul-Kee Park, Sung-Hye Park
- J Pathol Transl Med. 2021;55(2):102-111. Published online December 23, 2020
- DOI: https://doi.org/10.4132/jptm.2020.10.22
- 7,783 View
- 298 Download
- 21 Web of Science
- 18 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
CDKN2A is a tumor suppressor gene that encodes the cell cycle inhibitor protein p16. Homozygous deletion of the CDKN2A gene has been associated with shortened survival in isocitrate dehydrogenase (IDH)–mutant gliomas. This study aimed to analyze the prognostic value of p16 and to evaluate whether p16 immunohistochemical staining could be used as a prognostic marker to replace CDKN2A genotyping in diffuse gliomas.
Methods
p16 immunohistochemistry was performed on tissue microarrays of 326 diffuse gliomas with diagnoses that reflected IDH-mutations and 1p/19q codeletion status. The results were divided into three groups (negative, focal expression, overexpression) according to the presence and degree of p16 expression. Survival analysis was performed to assess the prognostic value of p16 expression.
Results
A loss of p16 expression predicted a significantly worse outcome in all glioma patients (n=326, p<.001), in the IDH-mutant glioma patients (n=103, p=.010), and in the IDH-mutant astrocytoma patients (n=73, p=.032). However, loss of p16 expression did not predict the outcome in the IDH-wildtype glioma patients (n=223, p=.121) or in the oligodendroglial tumor patients with the IDH-mutation and 1p/19q codeletion (n=30, p=.457). Multivariate analysis showed the association was still significant in the IDH-mutant glioma patients (p=.008; hazard ratio [HR], 2.637; 95% confidence interval [CI], 1.295 to 5.372) and in the IDH-mutant astrocytoma patients (p=.001; HR, 3.586; 95% CI, 1.649 to 7.801). Interestingly, patients who presented with tumors with p16 overexpression also had shorter survival times than did patients with tumors with p16 focal expression in the whole glioma (p< .001) and in IDH-mutant glioma groups. (p=.046).
Conclusions
This study suggests that detection of p16 expression by immunohistochemistry can be used as a useful surrogate test to predict prognosis, especially in IDH-mutant astrocytoma patients. -
Citations
Citations to this article as recorded by- A comparison of CDKN2A status in gliomas using different techniques: The loss of p16 as a surrogate marker
Arnault Tauziède-Espariat, Audrey Rousseau, Laetitia Basset, Raphaël Saffroy, Ana Cavillon, Amélie Lusque, Lauren Hasty, Alice Métais, Yvan Nicaise, Emmanuelle Uro-Coste, Pascale Varlet, Pascale Varlet, Arnault Tauziède-Espariat
Journal of Neuropathology & Experimental Neurology.2025;[Epub] CrossRef - Cell-Specific Vulnerability of Human Glioblastoma and Astrocytoma Cells to Mephedrone—An In Vitro Study
Marta Marszalek-Grabska, Marta Kinga Lemieszek, Michal Chojnacki, Sylwia Winiarczyk, Joanna Jakubowicz-Gil, Barbara Zarzyka, Jarosław Pawelec, Jolanta H. Kotlinska, Wojciech Rzeski, Waldemar A. Turski
Molecules.2025; 30(11): 2277. CrossRef - Revealing the role of necroptosis microenvironment: FCGBP + tumor-associated macrophages drive primary liver cancer differentiation towards cHCC-CCA or iCCA
Chun Wang, Cuimin Chen, Wenting Hu, Lili Tao, Jiakang Chen
Apoptosis.2024; 29(3-4): 460. CrossRef - FISH analysis reveals CDKN2A and IFNA14 co-deletion is heterogeneous and is a prominent feature of glioblastoma
Sofian Al Shboul, Shelagh Boyle, Ashita Singh, Tareq Saleh, Moath Alrjoub, Ola Abu Al Karsaneh, Amel Mryyian, Rand Dawoud, Sinem Gul, Shaden Abu Baker, Kathryn Ball, Ted Hupp, Paul M. Brennan
Brain Tumor Pathology.2024; 41(1): 4. CrossRef - p16 Expression in Laryngeal Squamous Cell Carcinoma: A Surrogate or Independent Prognostic Marker?
Roberto Gallus, Davide Rizzo, Giorgia Rossi, Luca Mureddu, Jacopo Galli, Alberto Artuso, Francesco Bussu
Pathogens.2024; 13(2): 100. CrossRef - CDKN2A/B deletion in IDH-mutant astrocytomas: An evaluation by Fluorescence in-situ hybridization
Manali Ranade, Sridhar Epari, Omshree Shetty, Sandeep Dhanavade, Sheetal Chavan, Ayushi Sahay, Arpita Sahu, Prakash Shetty, Aliasgar Moiyadi, Vikash Singh, Archya Dasgupta, Abhishek Chatterjee, Sadhana Kannan, Tejpal Gupta
Journal of Neuro-Oncology.2024; 167(1): 189. CrossRef - Molecular prognostication in grade 3 meningiomas and p16/MTAP immunohistochemistry for predicting CDKN2A/B status
Kira Tosefsky, Karina Chornenka Martin, Alexander D Rebchuk, Justin Z Wang, Farshad Nassiri, Amy Lum, Gelareh Zadeh, Serge Makarenko, Stephen Yip
Neuro-Oncology Advances.2024;[Epub] CrossRef - Insights into brain tumor diagnosis: exploring in situ hybridization techniques
E. D. Namiot, G. M. Zembatov, P. P. Tregub
Frontiers in Neurology.2024;[Epub] CrossRef - CDKN2A Homozygous Deletion Is a Stronger Predictor of Outcome than IDH1/2-Mutation in CNS WHO Grade 4 Gliomas
Sang Hyuk Lee, Tae Gyu Kim, Kyeong Hwa Ryu, Seok Hyun Kim, Young Zoon Kim
Biomedicines.2024; 12(10): 2256. CrossRef - Homozygous CDKN2A/B deletions in low- and high-grade glioma: a meta-analysis of individual patient data and predictive values of p16 immunohistochemistry testing
Darius Noack, Johannes Wach, Alonso Barrantes-Freer, Nils H. Nicolay, Erdem Güresir, Clemens Seidel
Acta Neuropathologica Communications.2024;[Epub] CrossRef - p16 Immunohistochemical Expression as a Surrogate Assessment of CDKN2A Alteration in Gliomas Leading to Prognostic Significances
Lucas Geyer, Thibaut Wolf, Marie-Pierre Chenard, Helene Cebula, Roland Schott, Georges Noel, Eric Guerin, Erwan Pencreach, Damien Reita, Natacha Entz-Werlé, Benoît Lhermitte
Cancers.2023; 15(5): 1512. CrossRef - P16 immunohistochemistry is a sensitive and specific surrogate marker for CDKN2A homozygous deletion in gliomas
Meenakshi Vij, Benjamin B. Cho, Raquel T. Yokoda, Omid Rashidipour, Melissa Umphlett, Timothy E. Richardson, Nadejda M. Tsankova
Acta Neuropathologica Communications.2023;[Epub] CrossRef -
CDKN2A mutations have equivalent prognostic significance to homozygous deletion in IDH-mutant astrocytoma
Raquel T Yokoda, William S Cobb, Raymund L Yong, John F Crary, Mariano S Viapiano, Jamie M Walker, Melissa Umphlett, Nadejda M Tsankova, Timothy E Richardson
Journal of Neuropathology & Experimental Neurology.2023; 82(10): 845. CrossRef - Efficient diagnosis of IDH-mutant gliomas: 1p/19qNET assesses 1p/19q codeletion status using weakly-supervised learning
Gi Jeong Kim, Tonghyun Lee, Sangjeong Ahn, Youngjung Uh, Se Hoon Kim
npj Precision Oncology.2023;[Epub] CrossRef - The Prognostic Significance of P16 Immunohistochemical Expression Pattern in Women with Invasive Ductal Breast Carcinoma
Alireza Rezaei, Navidreza Shayan, Saman Shirazinia, Sara Mollazadeh, Negin Ghiyasi-Moghaddam
Reports of Biochemistry and Molecular Biology.2023; 12(1): 83. CrossRef - Sporadic and Lynch syndrome-associated mismatch repair-deficient brain tumors
Hyunhee Kim, Ka Young Lim, Jin Woo Park, Jeongwan Kang, Jae Kyung Won, Kwanghoon Lee, Yumi Shim, Chul-Kee Park, Seung-Ki Kim, Seung-Hong Choi, Tae Min Kim, Hongseok Yun, Sung-Hye Park
Laboratory Investigation.2022; 102(2): 160. CrossRef - Simple approach for the histomolecular diagnosis of central nervous system gliomas based on 2021 World Health Organization Classification
Maher Kurdi, Rana H Moshref, Yousef Katib, Eyad Faizo, Ahmed A Najjar, Basem Bahakeem, Ahmed K Bamaga
World Journal of Clinical Oncology.2022; 13(7): 567. CrossRef - P16INK4A—More Than a Senescence Marker
Hasan Safwan-Zaiter, Nicole Wagner, Kay-Dietrich Wagner
Life.2022; 12(9): 1332. CrossRef
- A comparison of CDKN2A status in gliomas using different techniques: The loss of p16 as a surrogate marker
- A comparative prognostic performance of definitions of Crohn-like lymphoid reaction in colorectal carcinoma
- Younghoon Kim, Jeong Mo Bae, Jung Ho Kim, Nam-Yun Cho, Gyeong Hoon Kang
- J Pathol Transl Med. 2021;55(1):53-59. Published online November 27, 2020
- DOI: https://doi.org/10.4132/jptm.2020.10.06
- 4,915 View
- 140 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
The prognostic potential of Crohn-like lymphoid reaction (CLR) in colorectal carcinoma (CRC) has been investigated through the assessment of different criteria.
Methods
The prognostic impact of CLR was investigated in 636 CRC patients to compare methods from previously published articles. These methods included CLR measured by number of lymphoid aggregates (LAs) (CLR count), LA size greater than or equal to 1 mm (CLR size), CLR density with a cutoff value of 0.38, and subjective criteria as defined by intense CLR.
Results
In univariate survival analysis, CLR-positive CRC as defined by the four aforementioned methods was associated with better overall survival (OS) (hazard ratio [HR], 0.463; 95% confidence interval [CI], 0.305 to 0.702; p <.001; HR, 0.656; 95% CI, 0.411 to 1.046; p=.077; HR, 0.363; 95% CI, 0.197 to 0.669; p=.001; and HR, 0.433; 95% CI, 0.271 to 0.690; p<.001, respectively) and disease-free survival (DFS) (HR, 0.411; 95% CI, 0.304 to 0.639; p<.001; HR, 0.528; 95% CI, 0.340 to 0.821; p=.004; HR, 0.382; 95% CI, 0.226 to 0.645, p=.004; and HR, 0.501; 95% CI, 0.339 to 0.741; p<.001, respectively) than CLR-negative CRC, regardless of criteria with the exception of OS for CLR density. In multivariate analysis, two objective criteria (CLR count and CLR density) and one subjective criterion (intense CLR) for defining CLR were considered independent prognostic factors of OS and DFS in CRC patients.
Conclusions
CLR has similar traits regardless of criteria, but CLR-positivity should be defined by objective criteria for better reproducibility and prognostic value.
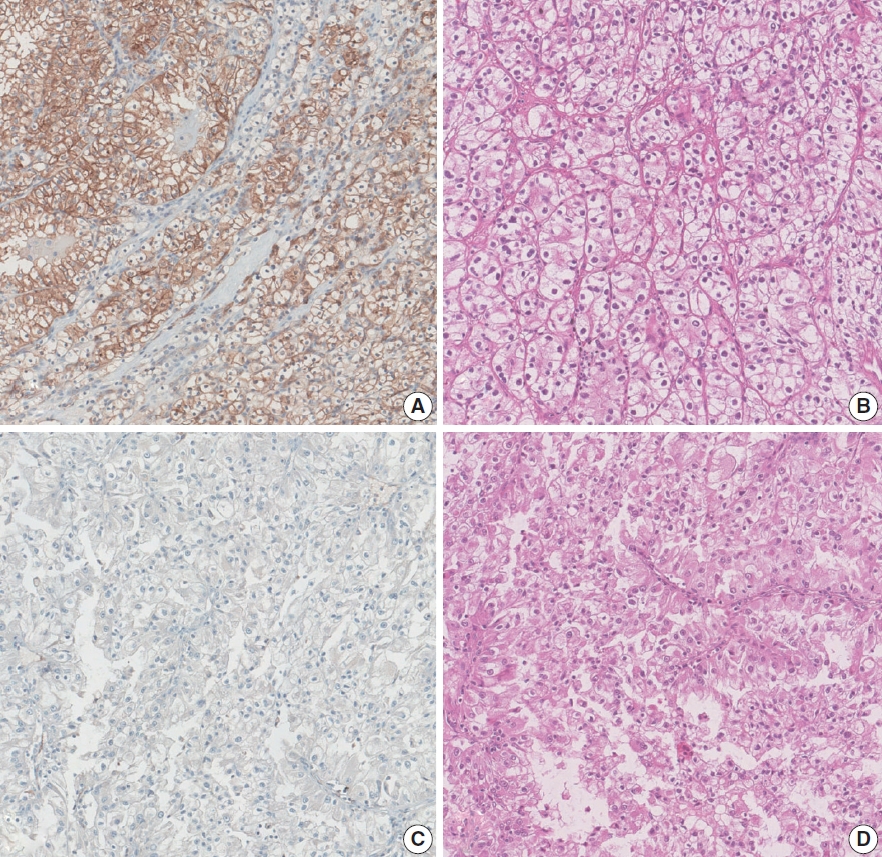
- Programmed death-ligand 1 expression and its correlation with clinicopathological parameters in gallbladder cancer
- Ji Hye Kim, Kyungbin Kim, Misung Kim, Young Min Kim, Jae Hee Suh, Hee Jeong Cha, Hye Jeong Choi
- J Pathol Transl Med. 2020;54(2):154-164. Published online February 10, 2020
- DOI: https://doi.org/10.4132/jptm.2019.11.13
- 8,383 View
- 167 Download
- 15 Web of Science
- 14 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Immunomodulatory therapies targeting the interaction between programmed cell death protein 1 and programmed death-ligand 1 (PD-L1) have become increasingly important in anticancer treatment. Previous research on the subject of this immune response has established an association with tumor aggressiveness and a poor prognosis in certain cancers. Currently, scant information is available on the relationship between PD-L1 expression and gallbladder cancer (GBC).
Methods
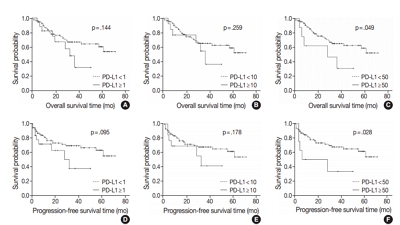
We investigated the expression of PD-L1 in 101 primary GBC cases to determine the potential association with prognostic impact. PD-L1 expression was immunohistochemically assessed using a single PD-L1 antibody (clone SP263). Correlations with clinicopathological parameters, overall survival (OS), or progression- free survival (PFS) were analyzed.
Results
PD-L1 expression in tumor cells at cutoff levels of 1%, 10%, and 50% was present in 18.8%, 13.8%, and 7.9% of cases. Our study showed that positive PD-L1 expression at any cutoff was significantly correlated with poorly differentiated histologic grade and the presence of lymphovascular invasion (p < .05). PD-L1 expression at cutoff levels of 10% and 50% was significantly positive in patients with perineural invasion, higher T categories, and higher pathologic stages (p < .05). Additionally, there was a significant association noted between PD-L1 expression at a cutoff level of 50% and worse OS or PFS (p = .049 for OS, p = .028 for PFS). Other poor prognostic factors included histologic grade, T category, N category, pathologic stage, lymphovascular invasion, perineural invasion, growth pattern, and margin of resection (p < .05).
Conclusions
The expression of PD-L1 in GBC varies according to cutoff level but is valuably associated with poor prognostic parameters and survival. Our study indicates that the overexpression of PD-L1 in GBC had a negative prognostic impact. -
Citations
Citations to this article as recorded by- PD-L1 Expression in Biliary Tract Cancer: Comparison Across Antibody Clones and Role as a Predictor of Response to Chemoimmunotherapy: A Meta-Analysis
Juan J. Juarez-Vignon Whaley, Soravis Osataphan, Ben Ponvilawan, Nipith Charoenngam, Mary Linton Peters
JCO Precision Oncology.2025;[Epub] CrossRef - Lacking Immunotherapy Biomarkers for Biliary Tract Cancer: A Comprehensive Systematic Literature Review and Meta-Analysis
Giorgio Frega, Fernando P. Cossio, Jesus M. Banales, Vincenzo Cardinale, Rocio I. R. Macias, Chiara Braconi, Angela Lamarca
Cells.2023; 12(16): 2098. CrossRef - Gallbladder carcinomas: review and updates on morphology, immunohistochemistry, and staging
Whayoung Lee, Vishal S. Chandan
Human Pathology.2023; 132: 149. CrossRef - Prognostic Relevance of PDL1 and CA19-9 Expression in Gallbladder Cancer vs. Inflammatory Lesions
Neetu Rawal, Supriya Awasthi, Nihar Ranjan Dash, Sunil Kumar, Prasenjit Das, Amar Ranjan, Anita Chopra, Maroof Ahmad Khan, Sundeep Saluja, Showket Hussain, Pranay Tanwar
Current Oncology.2023; 30(2): 1571. CrossRef - Identification of genes associated with gall bladder cell carcinogenesis: Implications in targeted therapy of gall bladder cancer
Ishita Ghosh, Ruma Dey Ghosh, Soma Mukhopadhyay
World Journal of Gastrointestinal Oncology.2023; 15(12): 2053. CrossRef - CD73 and PD-L1 as Potential Therapeutic Targets in Gallbladder Cancer
Lu Cao, Kim R. Bridle, Ritu Shrestha, Prashanth Prithviraj, Darrell H. G. Crawford, Aparna Jayachandran
International Journal of Molecular Sciences.2022; 23(3): 1565. CrossRef - Evolving Role of Immunotherapy in Advanced Biliary Tract Cancers
Sandra Kang, Bassel F. El-Rayes, Mehmet Akce
Cancers.2022; 14(7): 1748. CrossRef - Novel immune scoring dynamic nomograms based on B7-H3, B7-H4, and HHLA2: Potential prediction in survival and immunotherapeutic efficacy for gallbladder cancer
Chao Lv, Shukun Han, Baokang Wu, Zhiyun Liang, Yang Li, Yizhou Zhang, Qi Lang, Chongli Zhong, Lei Fu, Yang Yu, Feng Xu, Yu Tian
Frontiers in Immunology.2022;[Epub] CrossRef - PD-1 inhibitors plus nab-paclitaxel-containing chemotherapy for advanced gallbladder cancer in a second-line setting: A retrospective analysis of a case series
Sirui Tan, Jing Yu, Qiyue Huang, Nan Zhou, Hongfeng Gou
Frontiers in Oncology.2022;[Epub] CrossRef - Expression of HER2 and Mismatch Repair Proteins in Surgically Resected Gallbladder Adenocarcinoma
You-Na Sung, Sung Joo Kim, Sun-Young Jun, Changhoon Yoo, Kyu-Pyo Kim, Jae Hoon Lee, Dae Wook Hwang, Shin Hwang, Sang Soo Lee, Seung-Mo Hong
Frontiers in Oncology.2021;[Epub] CrossRef - Programmed Death Ligand-1 (PD-L1) Is an Independent Negative Prognosticator in Western-World Gallbladder Cancer
Thomas Albrecht, Fritz Brinkmann, Michael Albrecht, Anke S. Lonsdorf, Arianeb Mehrabi, Katrin Hoffmann, Yakup Kulu, Alphonse Charbel, Monika N. Vogel, Christian Rupp, Bruno Köhler, Christoph Springfeld, Peter Schirmacher, Stephanie Roessler, Benjamin Goep
Cancers.2021; 13(7): 1682. CrossRef - Immune Microenvironment in Gallbladder Adenocarcinomas
Pallavi A. Patil, Kara Lombardo, Weibiao Cao
Applied Immunohistochemistry & Molecular Morphology.2021; 29(8): 557. CrossRef - Molecular Targets and Emerging Therapies for Advanced Gallbladder Cancer
Matteo Canale, Manlio Monti, Ilario Giovanni Rapposelli, Paola Ulivi, Francesco Giulio Sullo, Giulia Bartolini, Elisa Tiberi, Giovanni Luca Frassineti
Cancers.2021; 13(22): 5671. CrossRef - Overview of current targeted therapy in gallbladder cancer
Xiaoling Song, Yunping Hu, Yongsheng Li, Rong Shao, Fatao Liu, Yingbin Liu
Signal Transduction and Targeted Therapy.2020;[Epub] CrossRef
- PD-L1 Expression in Biliary Tract Cancer: Comparison Across Antibody Clones and Role as a Predictor of Response to Chemoimmunotherapy: A Meta-Analysis
- Expression of female sex hormone receptors and its relation to clinicopathological characteristics and prognosis of lung adenocarcinoma
- Jin Hwan Lee, Han Kyeom Kim, Bong Kyung Shin
- J Pathol Transl Med. 2020;54(1):103-111. Published online November 13, 2019
- DOI: https://doi.org/10.4132/jptm.2019.10.12
- 6,887 View
- 141 Download
- 6 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Adenocarcinoma (ADC) of the lung exhibits different clinicopathological characteristics in men and women. Recent studies have suggested that these differences originate from the expression of female sex hormone receptors in tumor cells. The aim of the present study was to evaluate the immunohistochemical expression of female sex hormone receptors in lung ADC and determine the expression patterns in patients with different clinicopathological characteristics.
Methods
A total of 84 patients with lung ADC who underwent surgical resection and/or core biopsy were recruited for the present study. Immunohistochemical staining was performed for estrogen receptor α (ERα), estrogen receptor β (ERβ), progesterone receptor (PR), epidermal growth factor receptor (EGFR), EGFR E746- A750 del, and EGFR L858R using tissue microarray.
Results
A total of 39 (46.4%) ERα-positive, 71 (84.5%) ERβ-positive, and 46 (54.8%) PR-positive lung ADCs were identified. In addition, there were 81 (96.4%) EGFR-positive, 14 (16.7%) EGFR E746-A750 del–positive, and 34 (40.5%) EGFR L858R–positive cases. The expression of female sex hormone receptors was not significantly different in clinicopathologically different subsets of lung ADC.
Conclusions
Expression of female sex hormone receptors is not associated with the prognosis and clinicopathological characteristics of patients with lung ADC. -
Citations
Citations to this article as recorded by- Molecular characteristics of non-small cell lung cancer tissue based on quantitative indicators of progesterone receptors expression
I. P. Romanov, T. A. Bogush, A. M. Scherbakov, A. A. Alimov, E. A. Bogush, A. B. Ravcheeva, A. Lee, V. S. Kosorukov
Antibiot Khimioter = Antibiotics and Chemotherapy.2024; 69(1-2): 29. CrossRef - Genes Co-Expressed with ESR2 Influence Clinical Outcomes in Cancer Patients: TCGA Data Analysis
Julia Maria Lipowicz, Agnieszka Malińska, Michał Nowicki, Agnieszka Anna Rawłuszko-Wieczorek
International Journal of Molecular Sciences.2024; 25(16): 8707. CrossRef - Complex Differential Diagnosis between Primary Breast Cancer and Breast Metastasis from EGFR-Mutated Lung Adenocarcinoma: Case Report and Literature Review
Carmine Valenza, Francesca Maria Porta, Alessandra Rappa, Elena Guerini-Rocco, Giuseppe Viale, Massimo Barberis, Filippo de Marinis, Giuseppe Curigliano, Chiara Catania
Current Oncology.2021; 28(5): 3384. CrossRef - Development of a 15‐Gene Signature Model as a Prognostic Tool in Sex Hormone‐Dependent Cancers
Zhi Xia, Jian Xiao, Aibin Liu, Qiong Chen, Arumugam R. Jayakumar
BioMed Research International.2021;[Epub] CrossRef - Gender-specific aspects of epidemiology, molecular genetics and outcome: lung cancer
Nuria Mederos, Alex Friedlaender, Solange Peters, Alfredo Addeo
ESMO Open.2020; 5(Suppl 4): e000796. CrossRef
- Molecular characteristics of non-small cell lung cancer tissue based on quantitative indicators of progesterone receptors expression
- Clinicopathological Characterization and Prognostic Implication of SMAD4 Expression in Colorectal Carcinoma
- Seung-Yeon Yoo, Ji-Ae Lee, Yunjoo Shin, Nam-Yun Cho, Jeong Mo Bae, Gyeong Hoon Kang
- J Pathol Transl Med. 2019;53(5):289-297. Published online June 24, 2019
- DOI: https://doi.org/10.4132/jptm.2019.06.07
- 8,080 View
- 161 Download
- 8 Web of Science
- 9 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
SMAD family member 4 (SMAD4) has gained attention as a promising prognostic factor of colorectal cancer (CRC) as well as a key molecule to understand the tumorigenesis and progression of CRC.
Methods
We retrospectively analyzed 1,281 CRC cases immunohistochemically for their expression status of SMAD4, and correlated this status with clinicopathologic and molecular features of CRCs.
Results
A loss of nuclear SMAD4 was significantly associated with frequent lymphovascular and perineural invasion, tumor budding, fewer tumor-infiltrating lymphocytes, higher pT and pN category, and frequent distant metastasis. In contrast, tumors overexpressing SMAD4 showed a significant association with sporadic microsatellite instability. After adjustment for TNM stage, tumor differentiation, adjuvant chemotherapy, and lymphovascular invasion, the loss of SMAD4 was found to be an independent prognostic factor for worse 5-year progression-free survival (hazard ratio [HR], 1.27; 95% confidence interval [CI], 1.01 to 1.60; p=.042) and 7-year cancerspecific survival (HR, 1.45; 95% CI, 1.06 to 1.99; p=.022).
Conclusions
We confirmed the value of determining the loss of SMAD4 immunohistochemically as an independent prognostic factor for CRC in general. In addition, we identified some histologic and molecular features that might be clues to elucidate the role of SMAD4 in colorectal tumorigenesis and progression. -
Citations
Citations to this article as recorded by- Association between the expression of epithelial–mesenchymal transition (EMT)-related markers and oncologic outcomes of colorectal cancer
Mona Hany Emile, Sameh Hany Emile, Amr Awad El-Karef, Mohamed Awad Ebrahim, Ibrahim Eldosoky Mohammed, Dina Abdallah Ibrahim
Updates in Surgery.2024; 76(6): 2181. CrossRef - TGF-β and SMAD2/4 Expression in Nonmetastatic and Metastatic Colorectal Cancer Patients
Ainul Mardiah, Hendra Susanto, Sri Rahayu Lestari, A. Taufiq, H. Susanto, H. Nur, M. Diantoro, M. Aziz, N.A.N.N. Malek
BIO Web of Conferences.2024; 117: 01001. CrossRef - Unraveling Resistance to Immunotherapy in MSI-High Colorectal Cancer
Ronald Heregger, Florian Huemer, Markus Steiner, Alejandra Gonzalez-Martinez, Richard Greil, Lukas Weiss
Cancers.2023; 15(20): 5090. CrossRef - Association of β-Catenin, APC, SMAD3/4, Tp53, and Cyclin D1 Genes in Colorectal Cancer: A Systematic Review and Meta-Analysis
Hongfeng Yan, Fuquan Jiang, Jianwu Yang, Ying-Kun Xu
Genetics Research.2022; 2022: 1. CrossRef - Comprehensive genetic features of gastric mixed adenoneuroendocrine carcinomas and pure neuroendocrine carcinomas
Jiwon Koh, Soo Kyung Nam, Yoonjin Kwak, Gilhyang Kim, Ka‐Kyung Kim, Byung‐Chul Lee, Sang‐Hoon Ahn, Do Joong Park, Hyung‐Ho Kim, Kyoung Un Park, Woo Ho Kim, Hye Seung Lee
The Journal of Pathology.2021; 253(1): 94. CrossRef - Alterations of PTEN and SMAD4 methylation in diagnosis of breast cancer: implications of methyl II PCR assay
Menha Swellam, Entsar A. Saad, Shimaa Sabry, Adel Denewer, Camelia Abdel Malak, Amr Abouzid
Journal of Genetic Engineering and Biotechnology.2021; 19(1): 54. CrossRef - Molecular Characterization and Functional Analysis of Two Steroidogenic Genes TSPO and SMAD4 in Yellow Catfish
Fang Chen, Chong-Chao Zhong, Chang-Chun Song, Shu-Wei Chen, Yang He, Xiao-Ying Tan
International Journal of Molecular Sciences.2021; 22(9): 4505. CrossRef - SMAD7 and SMAD4 expression in colorectal cancer progression and therapy response
Jovana Rosic, Sandra Dragicevic, Marko Miladinov, Jovana Despotovic, Aleksandar Bogdanovic, Zoran Krivokapic, Aleksandra Nikolic
Experimental and Molecular Pathology.2021; 123: 104714. CrossRef - Actionable Potentials of Less Frequently Mutated Genes in Colorectal Cancer and Their Roles in Precision Medicine
Ryia Illani Mohd Yunos, Nurul Syakima Ab Mutalib, Francis Yew Fu Tieng, Nadiah Abu, Rahman Jamal
Biomolecules.2020; 10(3): 476. CrossRef
- Association between the expression of epithelial–mesenchymal transition (EMT)-related markers and oncologic outcomes of colorectal cancer

 E-submission
E-submission











 First
First Prev
Prev



