Search
- Page Path
- HOME > Search
- Histopathological characteristics of Epstein-Barr virus (EBV)–associated encephalitis and colitis in chronic active EBV infection
- Betty A Kasimo, James J Yahaya, Sun Och Yoon, Se Hoon Kim, Minsun Jung
- J Pathol Transl Med. 2025;59(3):188-194. Published online April 16, 2025
- DOI: https://doi.org/10.4132/jptm.2025.02.21
- 3,992 View
- 164 Download
-
 Abstract
Abstract
 PDF
PDF - Chronic active Epstein-Barr virus (CAEBV) can induce complications in various organs, including the brain and gastrointestinal tract. A 3-year-old boy was referred to the hospital with a history of fever and seizures for 15 days. A diagnosis of encephalitis based on computed tomography (CT) and magnetic resonance imaging findings and clinical correlation was made. Laboratory tests showed positive serology for Epstein-Barr virus (EBV) and negative for Rotavirus antigen and IgG and IgM antibodies for cytomegalovirus, herpes simplex virus, and varicella zoster virus, respectively. Abdominal CT showed diffuse wall thickening with fluid distension of small bowel loops, lower abdomen wall thickening, and a small amount of ascites. The biopsy demonstrated positive Epstein-Barr encoding region in situ hybridization in cells within the crypts and lamina propria. The patient was managed with steroids and hematopoietic stem cell transplantation (HSCT). This case showed histopathological characteristics of concurrent EBV-associated encephalitis and colitis in CAEBV infection. The three-step strategy of immunosuppressive therapy, chemotherapy, and allogeneic HSCT should be always be considered for prevention of disease progression.
- Uncommon granulomatous manifestation in Epstein-Barr virus–positive follicular dendritic cell sarcoma: a case report
- Henry Goh Di Shen, Yue Zhang, Wei Qiang Leow
- J Pathol Transl Med. 2025;59(2):133-138. Published online October 31, 2024
- DOI: https://doi.org/10.4132/jptm.2024.09.27
- 3,669 View
- 346 Download
- 6 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF - Hepatic Epstein-Barr virus–positive inflammatory follicular dendritic cell sarcoma (EBV+ IFDCS) represents a rare form of liver malignancy. The absence of distinct clinical and radiological characteristics, compounded by its rare occurrence, contributes to a challenging diagnosis. Here, we report a case of a 54-year-old Chinese female with a background of chronic hepatitis B virus treated with entecavir and complicated by advanced fibrosis presenting with a liver mass found on her annual surveillance ultrasound. Hepatectomy was performed under clinical suspicion of hepatocellular carcinoma. Immunomorphologic characteristics of the tumor were consistent with EBV+ IFDCS with distinct non-caseating granulomatous inflammation. Our case illustrates the importance of considering EBV+ IFDCS in the differential diagnosis of hepatic inflammatory lesions. Awareness of this entity and its characteristic features is essential for accurately diagnosing and managing this rare neoplasm.
-
Citations
Citations to this article as recorded by- Mesenchymal Tumors of the Liver: An Update Review
Joon Hyuk Choi, Swan N. Thung
Biomedicines.2025; 13(2): 479. CrossRef - EBV-positive inflammatory follicular dendritic cell sarcoma occurring in different organs: a case report and literature review
Wenhua Bai, Chunfang Hu, Zheng Zhu
Frontiers in Oncology.2025;[Epub] CrossRef - Spleen EBV-positive inflammatory follicular dendritic cell sarcoma: a case report and literature review
Yi Xiao, Lanlan Li, Xiumei Zhan, Juner Xu, Yewu Chen, Qiuchan Zhao, Yinghao Fu, Xian Luo, Huadi Chen, Hao Xu
Frontiers in Oncology.2025;[Epub] CrossRef - Epstein-Barr virus-positive inflammatory follicular dendritic cell sarcoma of the liver: clinical features, imaging findings and potential diagnostic clues
Gui-Ling Huang, Man-Qian Huang, Yu-Ting Zhang, Hui-Ning Huang, Hong-Tao Liu, Xiao-Qing Pei
Abdominal Radiology.2025;[Epub] CrossRef - Epstein‑Barr virus+ inflammatory follicular dendritic cell sarcoma with clonal immunoglobulin heavy chain gene rearrangement: A case report and literature review
Qian Ye, Juan Zhao, Jiao He, Weishan Zhang
Oncology Letters.2025; 31(2): 1. CrossRef - Primary hepatic follicular dendritic cell sarcoma: A case study and literature review
Junjie Zhu, Ying Liang, Li Zhang, Bingqi Li, Danfeng Zheng, Hangyan Wang
Journal of International Medical Research.2025;[Epub] CrossRef
- Mesenchymal Tumors of the Liver: An Update Review
- Intravascular NK/T-cell lymphoma: a case report and literature review
- Ji Min Na, Wookjae Jung, Minhye Kim, Yun-Hong Cheon, Jong Sil Lee, Dae Hyun Song, Jung Wook Yang
- J Pathol Transl Med. 2023;57(6):332-336. Published online November 14, 2023
- DOI: https://doi.org/10.4132/jptm.2023.10.30
- 5,700 View
- 235 Download
- 4 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF - Intravascular lymphoma is characterized by an exclusively intravascular distribution of tumor cells. Intravascular natural killer/T-cell lymphoma (IVNKTL) is extremely rare, highly aggressive, commonly Epstein-Barr virus (EBV)–positive, and predominantly affects the skin and central nervous system. Here we report a case of IVNKTL diagnosed in a 67-year-old female, presenting with persistent intermittent fever and skin rashes throughout the body. Incisional biopsy of an erythematous lesion on the chest exhibited aggregation of medium to large-sized atypical lymphoid cells confined to the lumen of small vessels that were positive for CD3, granzyme B, and CD56 on immunohistochemistry and EBV-encoded RNA in situ hybridization. EBV DNA was also detected in serum after diagnosis. With a review of 26 cases of IVNKTL to date, we suggest that active biopsy based on EBV DNA detection may facilitate early diagnosis of IVNKTL.
-
Citations
Citations to this article as recorded by- Mimicry in the vasculature: a review of diagnostic clues in cutaneous intravascular lymphoid proliferations
MA Faraz, S Tu Zahra, F Ocampo-Gonzalez, SC Shalin, Aadil Ahmed
Diagnostic Histopathology.2026;[Epub] CrossRef - Intravascular Lymphoma: A Unique Pattern Underlying a Protean Disease
Mario Della Mura, Joana Sorino, Filippo Emanuele Angiuli, Gerardo Cazzato, Francesco Gaudio, Giuseppe Ingravallo
Cancers.2025; 17(14): 2355. CrossRef - Cutaneous Intravascular Hematolymphoid Entities: A Review
Emily Hatheway Marshall, Bethany Brumbaugh, Allison Holt, Steven T. Chen, Mai P. Hoang
Diagnostics.2024; 14(7): 679. CrossRef - CD30- and CD56-positive atypical intravascular lymphocytes of the uterine cervix, mimicking intravascular lymphoma: A case report and review of the literature
Daisuke Yamashita, Munemichi Otani, Hayato Maruoka, Takuya Aoki, Shigeo Hara
Journal of Clinical and Experimental Hematopathology.2024; 64(4): 328. CrossRef
- Mimicry in the vasculature: a review of diagnostic clues in cutaneous intravascular lymphoid proliferations
- Clinicopathologic characterization of cervical metastasis from an unknown primary tumor: a multicenter study in Korea
- Miseon Lee, Uiree Jo, Joon Seon Song, Youn Soo Lee, Chang Gok Woo, Dong-Hoon Kim, Jung Yeon Kim, Sun Och Yoon, Kyung-Ja Cho
- J Pathol Transl Med. 2023;57(3):166-177. Published online May 10, 2023
- DOI: https://doi.org/10.4132/jptm.2023.04.12
- 6,145 View
- 171 Download
- 6 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
Research regarding cervical metastasis from an unknown primary tumor (CUP) according to human papillomavirus (HPV) and Epstein-Barr virus (EBV) status in Korea has been sporadic and small-scale. This study aims to analyze and understand the characteristics of CUP in Korea according to viral and p16 and p53 status through a multicenter study.
Methods
Ninety-five cases of CUP retrieved from six hospitals in Korea between January 2006 and December 2016 were subjected to high-risk HPV detection (DNA in situ hybridization [ISH] or real-time polymerase chain reaction), EBV detection (ISH), and immunohistochemistry for p16 and p53.
Results
CUP was HPV-related in 37 cases (38.9%), EBV-related in five cases (5.3%), and unrelated to HPV or EBV in 46 cases (48.4%). HPV-related CUP cases had the best overall survival (OS) (p = .004). According to the multivariate analysis, virus-unrelated disease (p = .023) and longer smoking duration (p < .005) were prognostic factors for poor OS. Cystic change (p = .016) and basaloid pattern (p < .001) were more frequent in HPV-related cases, and lymphoepithelial lesion was frequent in EBV-related cases (p = .010). There was no significant association between viral status and p53 positivity (p = .341), smoking status (p = .728), or smoking duration (p = .187). Korean data differ from Western data in the absence of an association among HPV, p53 positivity, and smoking history.
Conclusions
Virus-unrelated CUP in Korea had the highest frequency among all CUP cases. HPV-related CUP is similar to HPV-mediated oropharyngeal cancer and EBVrelated CUP is similar to nasopharyngeal cancer in terms of characteristics, respectively. -
Citations
Citations to this article as recorded by- Differenzierung von benignen und malignen Halszysten – eine diagnostische Herausforderung
Christina Sauter, Matthias Sand, Karim Plath, Michaela Maria Plath
Laryngo-Rhino-Otologie.2025; 104(05): 296. CrossRef - Unlocking the Hidden: Advancing Imaging Techniques in Diagnosing Cancers of Unknown Primary in the Head and Neck Region
Daniela Messineo, Filippo Valentini, Giovanni Francesco Niccolini, Federica Zoccali, Francesca Ripari, Enrico Marotta, Marcello Caratozzolo, Pasquale Frisina
Applied Sciences.2025; 15(4): 2194. CrossRef - Characterization of undifferentiated carcinoma of the salivary gland: clinicopathological and immunohistochemical analyses in comparison with lymphoepithelial carcinoma
Sangjoon Choi, Gyuheon Choi, Hee Jin Lee, Joon Seon Song, Yoon Se Lee, Seung-Ho Choi, Kyung-Ja Cho
Journal of Pathology and Translational Medicine.2025; 59(6): 361. CrossRef - Management of squamous cell carcinoma of unknown primary in the head and neck: current evidence-based diagnostic and treatment strategies
Marcel Kloppenburg, Matthias Santer, Lukas Schmutzler, Felix Johnson, Benedikt Hofauer, Teresa Steinbichler
memo - Magazine of European Medical Oncology.2025;[Epub] CrossRef - Expansion of tumor-infiltrating lymphocytes from head and neck squamous cell carcinoma to assess the potential of adoptive cell therapy
Sangjoon Choi, Mofazzal Hossain, Hyun Lee, Jina Baek, Hye Seon Park, Chae-Lyul Lim, DoYeon Han, Taehyun Park, Jong Hyeok Kim, Gyungyub Gong, Mi-Na Kweon, Hee Jin Lee
Cancer Immunology, Immunotherapy.2024;[Epub] CrossRef
- Differenzierung von benignen und malignen Halszysten – eine diagnostische Herausforderung
- Unsuspected systemic Epstein-Barr virus–positive T-cell lymphoma of childhood diagnosed at autopsy in a potential homicide case
- Daniel J. Robbins, Erik A. Ranheim, Jamie E. Kallan
- J Pathol Transl Med. 2023;57(2):123-127. Published online December 22, 2022
- DOI: https://doi.org/10.4132/jptm.2022.10.31
- 5,576 View
- 188 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF - Systemic Epstein-Barr virus (EBV)–positive T-cell lymphoma of childhood (SETLC) is a rare, rapidly progressive, and often fatal disease of children and young adults characterized by monoclonal expansion of EBV-positive T cells in tissues or peripheral blood following infection with EBV. Its distinction from other EBV-positive T-cell lymphoproliferative disorders with overlapping features can be difficult, and particular diagnostic features may not be manifest until autopsy examination. We present the case of a 10-year-old boy with significant disability due to remote traumatic brain injury following non-accidental head trauma who died unexpectedly at home. Given the history of physical abuse and the potential for homicide charges, significant medicolegal implications arose with this case. Pathologic investigation ultimately revealed conclusive diagnostic features of SETLC including extensive proliferation of EBV-positive T cells in multiple organs. A natural manner of death was confirmed, thereby excluding delayed homicide related to complications of non-accidental head trauma.
-
Citations
Citations to this article as recorded by- The ‘Oma’s of the Gammas—Cancerogenesis by γ-Herpesviruses
Anwesha Banerjee, Debashree Dass, Soumik Mukherjee, Mollina Kaul, R. Harshithkumar, Parikshit Bagchi, Anupam Mukherjee
Viruses.2024; 16(12): 1928. CrossRef
- The ‘Oma’s of the Gammas—Cancerogenesis by γ-Herpesviruses
- Tumor immune response and immunotherapy in gastric cancer
- Yoonjin Kwak, An Na Seo, Hee Eun Lee, Hye Seung Lee
- J Pathol Transl Med. 2020;54(1):20-33. Published online November 1, 2019
- DOI: https://doi.org/10.4132/jptm.2019.10.08
- 17,976 View
- 748 Download
- 72 Web of Science
- 67 Crossref
-
 Abstract
Abstract
 PDF
PDF - Remarkable developments in immuno-oncology have changed the landscape of gastric cancer (GC) treatment. Because immunotherapy intervenes with tumor immune response rather than directly targeting tumor cells, it is important to develop a greater understanding of tumor immunity. This review paper summarizes the tumor immune reaction and immune escape mechanisms while focusing on the role of T cells and their co-inhibitory signals, such as the immune checkpoint molecules programmed death-1 and programmed deathligand 1 (PD-L1). This paper also describes past clinical trials of immunotherapy for patients with GC and details their clinical implications. Strong predictive markers are essential to improve response to immunotherapy. Microsatellite instability, Epstein-Barr virus, PD-L1 expression, and tumor mutational burden are now regarded as potent predictive markers for immunotherapy in patients with GC. Novel immunotherapy and combination therapy targeting new immune checkpoint molecules such as lymphocyte-activation gene 3, T cell immunoglobulin, and mucin domain containing-3, and indoleamine 2,3-dioxygenase have been suggested, and trials are ongoing to evaluate their safety and efficacy. Immunotherapy is an important treatment option for patients with GC and has great potential for improving patient outcome, and further research in immuno-oncology should be carried out.
-
Citations
Citations to this article as recorded by- Using glucocorticoid receptor-related genes to create and validate a survival model predicting gastric cancer
Ke Guo, Ping Huang, Jiasheng Zhang, Bo Zhang, Linyang Li, Jiaxin Li
Computational Biology and Chemistry.2026; 120: 108726. CrossRef - Mechanisms of Metabolic Reprogramming Regulating Immunosuppression in the Gastric Cancer Tumor Microenvironment
Wenting Dong, Xuepeng Qian, Honglin Liu, Jinhai Huo, Weiming Wang
Biomolecules.2026; 16(1): 160. CrossRef - FOXP3+/CD8+ ratio associated with aggressive behavior in RUNX3‐methylated diffuse esophagogastric junction tumor
Suguru Maruyama, Yu Imamura, Tasuku Toihata, Ikumi Haraguchi, Manabu Takamatsu, Makiko Yamashita, Yuichiro Nakashima, Eiji Oki, Kenichi Taguchi, Manabu Yamamoto, Shinji Mine, Akihiko Okamura, Jun Kanamori, Souya Nunobe, Takeshi Sano, Shigehisa Kitano, Tet
Cancer Science.2025; 116(1): 178. CrossRef - Immune biomarkers and predictive signatures in gastric cancer: Optimizing immunotherapy responses
Sundaram Vickram, Shofia Saghya Infant, S. Manikandan, D. Jenila Rani, C.M. Mathan Muthu, Hitesh Chopra
Pathology - Research and Practice.2025; 265: 155743. CrossRef - Korean Practice Guidelines for Gastric Cancer 2024: An Evidence-based, Multidisciplinary Approach (Update of 2022 Guideline)
In-Ho Kim, Seung Joo Kang, Wonyoung Choi, An Na Seo, Bang Wool Eom, Beodeul Kang, Bum Jun Kim, Byung-Hoon Min, Chung Hyun Tae, Chang In Choi, Choong-kun Lee, Ho Jung An, Hwa Kyung Byun, Hyeon-Su Im, Hyung-Don Kim, Jang Ho Cho, Kyoungjune Pak, Jae-Joon Kim
Journal of Gastric Cancer.2025; 25(1): 5. CrossRef - Targeted therapy and immunotherapy for gastric cancer: rational strategies, novel advancements, challenges, and future perspectives
Dong Luo, Yunmei Liu, Zhengmao Lu, Lei Huang
Molecular Medicine.2025;[Epub] CrossRef - Prognostic value of the triglyceride-glucose index in gastric cancer
Tugce Eskazan, Suat Saribas, Bekir Kocazeybek
World Journal of Gastroenterology.2025;[Epub] CrossRef - Deciphering the dual role of autophagy in gastric cancer and gastroesophageal junction cancer: from tumor suppression to cancer progression
Lili Lei, Junling Zhang, Ran Wei, Bingqi Dong, Xin Wang, Ying Zhou
Discover Oncology.2025;[Epub] CrossRef - Comparison of clinicopathological parameters with the presence of Epstein–Barr virus and the absence of DNA mismatch repair proteins in gastric adenocarcinomas
Özge Eyeoğlu, Serra Kayaçetin
Asian Biomedicine.2025; 19(2): 86. CrossRef - The impact of combined immunotherapy on the cellular composition of the tumor microenvironment in patients with gastric carcinoma
L.A. Tashireva, A.Yu. Kalinchuk, D.M. Loos, E.A. Tsarenkova, A.V. Avgustinovich, S.G. Afanas’ev, S.V. Vtorushin
Russian Journal of Archive of Pathology.2025; 87(4): 24. CrossRef - Immunotherapy in gastric adenocarcinoma – a rapidly evolving treatment landscape
Yang Wang, Geoffrey Chong
Critical Reviews in Oncology/Hematology.2025; 216: 104941. CrossRef - Ubiquilin-4 induces immune escape in gastric cancer by activating the notch signaling pathway
Quan Jiang, Hao Chen, Shixin Zhou, Tao Zhu, Wenshuai Liu, Hao Wu, Yong Zhang, Fenglin Liu, Yihong Sun
Cellular Oncology.2024; 47(1): 303. CrossRef - Expression and prognostic value of APOBEC2 in gastric adenocarcinoma and its association with tumor-infiltrating immune cells
Lipan Wei, Xiuqian Wu, Lan Wang, Ling Chen, Xuejun Wu, Tiantian Song, Yuanyuan Wang, Wenjun Chang, Aizhen Guo, Yongdong Niu, Haihua Huang
BMC Cancer.2024;[Epub] CrossRef - Identification and characterization of CLEC11A and its derived immune signature in gastric cancer
Qing Zheng, Zhenqi Gong, Baizhi Li, Runzi Cheng, Weican Luo, Cong Huang, Huaiming Wang
Frontiers in Immunology.2024;[Epub] CrossRef - Cervical cancer subtype identification and model building based on lipid metabolism and post-infection microenvironment immune landscape
Yongzhi Chen, Rongjie Cui, Dun Xiong, Yuan Zhao, Jianyu Pang, Samina Gul, Qi Qi, Yuheng Tang, Xuhong Zhou, Wenru Tang
Heliyon.2024; 10(9): e30746. CrossRef - Systematic Analysis of Tumor Stem Cell-related Gene Characteristics
to Predict the PD-L1 Immunotherapy and Prognosis of Gastric
Cancer
Chenchen Wang, Ying Chen, Ru Zhou, Ya’nan Yang, Yantian Fang
Current Medicinal Chemistry.2024; 31(17): 2467. CrossRef - Comprehensive landscape of m6A regulator-related gene patterns and tumor microenvironment infiltration characterization in gastric cancer
Bin Peng, Yinglin Lin, Gao Yi, Mingzhen Lin, Yao Xiao, Yezhenghong Qiu, Wenxia Yao, Xinke Zhou, Zhaoyu Liu
Scientific Reports.2024;[Epub] CrossRef - Distinctive Phenotypic and Microenvironmental Characteristics of Neuroendocrine Carcinoma and Adenocarcinoma Components in Gastric Mixed Adenoneuroendocrine Carcinoma
Yoonjin Kwak, Soo Kyung Nam, Yujun Park, Yun-Suhk Suh, Sang-Hoon Ahn, Seong-Ho Kong, Do Joong Park, Hyuk-Joon Lee, Hyung-Ho Kim, Han-Kwang Yang, Hye Seung Lee
Modern Pathology.2024; 37(10): 100568. CrossRef - Computed tomography-detected extramural venous invasion-related gene signature: a potential negative biomarker of immune checkpoint inhibitor treatment in patients with gastric cancer
Hao Yang, Xinyi Gou, Caizhen Feng, Yinli Zhang, Fan Chai, Nan Hong, Yingjiang Ye, Yi Wang, Bo Gao, Jin Cheng
Journal of Translational Medicine.2023;[Epub] CrossRef - A standardized pathology report for gastric cancer: 2nd edition
Young Soo Park, Myeong-Cherl Kook, Baek-hui Kim, Hye Seung Lee, Dong-Wook Kang, Mi-Jin Gu, Ok Ran Shin, Younghee Choi, Wonae Lee, Hyunki Kim, In Hye Song, Kyoung-Mee Kim, Hee Sung Kim, Guhyun Kang, Do Youn Park, So-Young Jin, Joon Mee Kim, Yoon Jung Choi,
Journal of Pathology and Translational Medicine.2023; 57(1): 1. CrossRef - A Standardized Pathology Report for Gastric Cancer: 2nd Edition
Young Soo Park, Myeong-Cherl Kook, Baek-hui Kim, Hye Seung Lee, Dong-Wook Kang, Mi-Jin Gu, Ok Ran Shin, Younghee Choi, Wonae Lee, Hyunki Kim, In Hye Song, Kyoung-Mee Kim, Hee Sung Kim, Guhyun Kang, Do Youn Park, So-Young Jin, Joon Mee Kim, Yoon Jung Choi,
Journal of Gastric Cancer.2023; 23(1): 107. CrossRef - Korean Practice Guidelines for Gastric Cancer 2022: An Evidence-based, Multidisciplinary Approach
Tae-Han Kim, In-Ho Kim, Seung Joo Kang, Miyoung Choi, Baek-Hui Kim, Bang Wool Eom, Bum Jun Kim, Byung-Hoon Min, Chang In Choi, Cheol Min Shin, Chung Hyun Tae, Chung sik Gong, Dong Jin Kim, Arthur Eung-Hyuck Cho, Eun Jeong Gong, Geum Jong Song, Hyeon-Su Im
Journal of Gastric Cancer.2023; 23(1): 3. CrossRef - Could Toll-like Receptor 2 Serve as Biomarker to Detect Advanced Gastric Cancer?
Marek Majewski, Kamil Torres, Paulina Mertowska, Sebastian Mertowski, Izabela Korona-Głowniak, Jan Korulczyk, Witold Zgodziński, Ewelina Grywalska
International Journal of Molecular Sciences.2023; 24(6): 5824. CrossRef - Research Progress of Immunotherapy for Gastric Cancer
Zhipeng Zhang, Ningning Liu, Mingyu Sun
Technology in Cancer Research & Treatment.2023;[Epub] CrossRef - Case Report: A rare synchronous multiple gastric carcinoma achieved progression-free disease through NGS-guided serial treatment
Xinyi Shao, Jin Yin, Di Wang, Erjiong Huang, Yini Zhang, Jiani C. Yin, Chen Huang, Hao Wu, Xiaoli Wu
Frontiers in Oncology.2023;[Epub] CrossRef - Artificial Intelligence-Enabled Gastric Cancer Interpretations
Mustafa Yousif, Liron Pantanowitz
Surgical Pathology Clinics.2023; 16(4): 673. CrossRef - The Optimal Tumor Mutational Burden Cutoff Value as a Novel Marker for Predicting the Efficacy of Programmed Cell Death-1 Checkpoint Inhibitors in Advanced Gastric Cancer
Jae Yeon Jang, Youngkyung Jeon, Sun Young Jeong, Sung Hee Lim, Won Ki Kang, Jeeyun Lee, Seung Tae Kim
Journal of Gastric Cancer.2023; 23(3): 476. CrossRef - Biomarkers for Predicting Response to Personalized Immunotherapy in Gastric Cancer
Moonsik Kim, Ji Yun Jeong, An Na Seo
Diagnostics.2023; 13(17): 2782. CrossRef - The Prognostic Value of the Prognostic Nutritional Index in Patients with Advanced or Metastatic Gastric Cancer Treated with Immunotherapy
Yuting Pan, Yue Ma, Guanghai Dai
Nutrients.2023; 15(19): 4290. CrossRef - Molecular classification of gastric cancer predicts survival in patients undergoing radical gastrectomy based on project HOPE
Kenichiro Furukawa, Keiichi Hatakeyama, Masanori Terashima, Takeshi Nagashima, Kenichi Urakami, Keiichi Ohshima, Akifumi Notsu, Takashi Sugino, Taisuke Yagi, Keiichi Fujiya, Satoshi Kamiya, Makoto Hikage, Yutaka Tanizawa, Etsuro Bando, Yae Kanai, Yasuto A
Gastric Cancer.2022; 25(1): 138. CrossRef - Immunotherapy for Gastric Cancer: A 2021 Update
Christo Kole, Nikolaos Charalampakis, Sergios Tsakatikas, Nikolaos-Iasonas Kouris, George Papaxoinis, Michalis V Karamouzis, Anna Koumarianou, Dimitrios Schizas
Immunotherapy.2022; 14(1): 41. CrossRef - The immune microenvironment in gastric adenocarcinoma
Yana Zavros, Juanita L. Merchant
Nature Reviews Gastroenterology & Hepatology.2022; 19(7): 451. CrossRef - Immunomodulation by Gut Microbiome on Gastrointestinal Cancers: Focusing on Colorectal Cancer
Raghad Khalid AL-Ishaq, Lenka Koklesova, Peter Kubatka, Dietrich Büsselberg
Cancers.2022; 14(9): 2140. CrossRef - An Immunity-Associated lncRNA Signature for Predicting Prognosis in Gastric Adenocarcinoma
Xiaowen Zhao, Pingfan Wu, Dongling Liu, Changtian Li, Ling Xue, Zhe Liu, Meng Zhu, Jie Yang, Ziyi Chen, Yaling Li, Yali She, Kathiravan Srinivasan
Journal of Healthcare Engineering.2022; 2022: 1. CrossRef - RNA modification writers influence tumor microenvironment in gastric cancer and prospects of targeted drug therapy
Peng Song, Sheng Zhou, Xiaoyang Qi, Yuwen Jiao, Yu Gong, Jie Zhao, Haojun Yang, Zhifen Qian, Jun Qian, Liming Tang
Journal of Bioinformatics and Computational Biology.2022;[Epub] CrossRef - Identification of the three subtypes and the prognostic characteristics of stomach adenocarcinoma: analysis of the hypoxia-related long non-coding RNAs
Zehua Fan, Yanqun Wang, Rong Niu
Functional & Integrative Genomics.2022; 22(5): 919. CrossRef - Complete Response of High Microsatellite Instability Gastric Cancer and Synchronous Microsatellite Stability Rectal Cancer
Zachary E Hunzeker, Pooja Bhakta, Sindusha R Gudipally, Sri Bharathi Kavuri, Rohit Venkatesan, Chukwuyejulumafor Nwanze
Cureus.2022;[Epub] CrossRef - Immune Profiling in Gastric Cancer Reveals the Dynamic Landscape of Immune Signature Underlying Tumor Progression
Yuhan Wei, Jianwei Zhang, Xueke Fan, Zhi Zheng, Xiaoyue Jiang, Dexi Chen, Yuting Lu, Yingrui Li, Miao Wang, Min Hu, Qi Du, Liuting Yang, Hongzhong Li, Yi Xiao, Yongfu Li, Jiangtao Jin, Deying Wang, Xiangliang Yuan, Qin Li
Frontiers in Immunology.2022;[Epub] CrossRef - Tumor vessel normalization and immunotherapy in gastric cancer
Xianzhe Yu, Shan He, Jian Shen, Qiushi Huang, Peng Yang, Lin Huang, Dan Pu, Li Wang, Lu Li, Jinghua Liu, Zelong Liu, Lingling Zhu
Therapeutic Advances in Medical Oncology.2022;[Epub] CrossRef - FN1 is a prognostic biomarker and correlated with immune infiltrates in gastric cancers
Han Wang, Junchang Zhang, Huan Li, Hong Yu, Songyao Chen, Shuhao Liu, Changhua Zhang, Yulong He
Frontiers in Oncology.2022;[Epub] CrossRef - Molecular Pathology of Gastric Cancer
Moonsik Kim, An Na Seo
Journal of Gastric Cancer.2022; 22(4): 264. CrossRef - Bioinformatics Analysis and Structure of Gastric Cancer Prognosis Model Based on Lipid Metabolism and Immune Microenvironment
Yongzhi Chen, Hongjun Yuan, Qian Yu, Jianyu Pang, Miaomiao Sheng, Wenru Tang
Genes.2022; 13(9): 1581. CrossRef - Clinical implications of interleukins-31, 32, and 33 in gastric cancer
Qing-Hua Liu, Ji-Wei Zhang, Lei Xia, Steven G Wise, Brett David Hambly, Kun Tao, Shi-San Bao
World Journal of Gastrointestinal Oncology.2022; 14(9): 1808. CrossRef - Microbiota and the Immune System—Actors in the Gastric Cancer Story
Marek Majewski, Paulina Mertowska, Sebastian Mertowski, Konrad Smolak, Ewelina Grywalska, Kamil Torres
Cancers.2022; 14(15): 3832. CrossRef - Bioinformatics and Experimental Analyses Reveal MAP4K4 as a Potential Marker for Gastric Cancer
Junping Zhang, Xiaoping Cai, Weifeng Cui, Zheng Wei
Genes.2022; 13(10): 1786. CrossRef - Common strategies for effective immunotherapy of gastroesophageal cancers using immune checkpoint inhibitors
Shuang Ma, Fei Chen
Pathology - Research and Practice.2022; 238: 154110. CrossRef - High-level of intratumoral GITR+ CD4 T cells associate with poor prognosis in gastric cancer
Shouyu Ke, Feng Xie, Yixian Guo, Jieqiong Chen, Zeyu Wang, Yimeng Yu, Haigang Geng, Danhua Xu, Xu Liu, Xiang Xia, Fengrong Yu, Chunchao Zhu, Zizhen Zhang, Gang Zhao, Bin Li, Wenyi Zhao
iScience.2022; 25(12): 105529. CrossRef - Characteristics of Adenosine-to-Inosine RNA editing-based subtypes and novel risk score for the prognosis and drug sensitivity in stomach adenocarcinoma
Jingjing Pan, Xinyuan Gu, Jing Luo, Xinye Qian, Qiang Gao, Tianjie Li, Longying Ye, Chenlu Li
Frontiers in Cell and Developmental Biology.2022;[Epub] CrossRef - Inhibition of NF‐κB is required for oleanolic acid to downregulate PD‐L1 by promoting DNA demethylation in gastric cancer cells
Xirong Lu, Yuyi Li, Wei Yang, Minghao Tao, Yanmiao Dai, Jinkang Xu, Qianfei Xu
Journal of Biochemical and Molecular Toxicology.2021;[Epub] CrossRef - Prognostic Value of C-Reactive Protein to Albumin Ratio in Gastric Cancer: A Meta-Analysis
Liang Yue, Yi Lu, Yulin Li, Yilin Wang
Nutrition and Cancer.2021; 73(10): 1864. CrossRef - Immunogenic characteristics of microsatellite instability‐low esophagogastric junction adenocarcinoma based on clinicopathological, molecular, immunological and survival analyses
Yu Imamura, Tasuku Toihata, Ikumi Haraguchi, Yoko Ogata, Manabu Takamatsu, Aya Kuchiba, Norio Tanaka, Osamu Gotoh, Seiichi Mori, Yuichiro Nakashima, Eiji Oki, Masaki Mori, Yoshinao Oda, Kenichi Taguchi, Manabu Yamamoto, Masaru Morita, Naoya Yoshida, Hideo
International Journal of Cancer.2021; 148(5): 1260. CrossRef - Two Similar Signatures for Predicting the Prognosis and Immunotherapy Efficacy of Stomach Adenocarcinoma Patients
Taohua Yue, Shuai Zuo, Jing Zhu, Shihao Guo, Zhihao Huang, Jichang Li, Xin Wang, Yucun Liu, Shanwen Chen, Pengyuan Wang
Frontiers in Cell and Developmental Biology.2021;[Epub] CrossRef - Tumor microenvironment characterization in stage IV gastric cancer
Feng Yang, Zhenbao Wang, Xianxue Zhang
Bioscience Reports.2021;[Epub] CrossRef - E2F2 inhibition induces autophagy via the PI3K/Akt/mTOR pathway in gastric cancer
Hui Li, Shufen Zhao, Liwei Shen, Peige Wang, Shihai Liu, Yingji Ma, Zhiwei Liang, Gongjun Wang, Jing Lv, Wensheng Qiu
Aging.2021; 13(10): 13626. CrossRef - Chemoradiation induces upregulation of immunogenic cell death-related molecules together with increased expression of PD-L1 and galectin-9 in gastric cancer
S. H. Petersen, L. F. Kua, S. Nakajima, W. P. Yong, K. Kono
Scientific Reports.2021;[Epub] CrossRef - Establishment of an Immune Cell Infiltration Score to Help Predict the Prognosis and Chemotherapy Responsiveness of Gastric Cancer Patients
Quan Jiang, Jie Sun, Hao Chen, Chen Ding, Zhaoqing Tang, Yuanyuan Ruan, Fenglin Liu, Yihong Sun
Frontiers in Oncology.2021;[Epub] CrossRef - Microsatellite instability in Gastric Cancer: Between lights and shadows
Elisabetta Puliga, Simona Corso, Filippo Pietrantonio, Silvia Giordano
Cancer Treatment Reviews.2021; 95: 102175. CrossRef - Survival-associated alternative splicing events interact with the immune microenvironment in stomach adenocarcinoma
Zai-Sheng Ye, Miao Zheng, Qin-Ying Liu, Yi Zeng, Sheng-Hong Wei, Yi Wang, Zhi-Tao Lin, Chen Shu, Qiu-Hong Zheng, Lu-Chuan Chen
World Journal of Gastroenterology.2021; 27(21): 2871. CrossRef - Immunotherapy of gastric cancer: Past, future perspective and challenges
Jun Xie, Liping Fu, Li Jin
Pathology - Research and Practice.2021; 218: 153322. CrossRef - Clinicopathologic and Prognostic Association of GRP94 Expression in Colorectal Cancer with Synchronous and Metachronous Metastases
Sumi Yun, Sukmook Lee, Ho-Young Lee, Hyeon Jeong Oh, Yoonjin Kwak, Hye Seung Lee
International Journal of Molecular Sciences.2021; 22(13): 7042. CrossRef - Injectable shear-thinning polylysine hydrogels for localized immunotherapy of gastric cancer through repolarization of tumor-associated macrophages
Yan Yang, Yang Yang, Meili Chen, Jianquan Chen, Jinyan Wang, Yajun Ma, Hanqing Qian
Biomaterials Science.2021; 9(19): 6597. CrossRef - Correlation between LRP1B Mutations and Tumor Mutation Burden in Gastric Cancer
Sizhe Hu, Xiaokang Zhao, Feng Qian, Cancan Jin, Kaishun Hou, Tao Huang
Computational and Mathematical Methods in Medicine.2021; 2021: 1. CrossRef - Comprehensive Analysis to Identify MAGEA3 Expression Correlated With Immune Infiltrates and Lymph Node Metastasis in Gastric Cancer
Jinji Jin, Jianxin Tu, Jiahuan Ren, Yiqi Cai, Wenjing Chen, Lifang Zhang, Qiyu Zhang, Guanbao Zhu
Frontiers in Oncology.2021;[Epub] CrossRef - Effect of P2X7 receptor on tumorigenesis and its pharmacological properties
Wen-jun Zhang, Ce-gui Hu, Zheng-ming Zhu, Hong-liang Luo
Biomedicine & Pharmacotherapy.2020; 125: 109844. CrossRef - Current status and future potential of predictive biomarkers for immune checkpoint inhibitors in gastric cancer
Byung Woog Kang, Ian Chau
ESMO Open.2020; 5(4): e000791. CrossRef - Is Ramucirumab Still the Only Second-Line Treatment in Metastatic Gastric Cancer?
Khalil El Gharib, Hampig Raphael Kourie
Pharmacogenomics.2020; 21(17): 1203. CrossRef - Deep Learning Predicts Underlying Features on Pathology Images with Therapeutic Relevance for Breast and Gastric Cancer
Renan Valieris, Lucas Amaro, Cynthia Aparecida Bueno de Toledo Osório, Adriana Passos Bueno, Rafael Andres Rosales Mitrowsky, Dirce Maria Carraro, Diana Noronha Nunes, Emmanuel Dias-Neto, Israel Tojal da Silva
Cancers.2020; 12(12): 3687. CrossRef
- Using glucocorticoid receptor-related genes to create and validate a survival model predicting gastric cancer
- Multistaining Optimization for Epstein-Barr Virus–Encoded RNA In Situ Hybridization and Immunohistochemistry of Formalin-Fixed Paraffin-Embedded Tissues Using an Automated Immunostainer
- Jae Nam Ko, Jin Kyoung Jung, Yun Ik Park, Hwa Jeong Shin, Jooryung Huh, Sol Back, Yu Jin Kim, Jae Ho Kim, Heounjeong Go
- J Pathol Transl Med. 2019;53(5):317-326. Published online August 27, 2019
- DOI: https://doi.org/10.4132/jptm.2019.08.06
- 8,885 View
- 128 Download
- 3 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
Single staining is commonly performed for practical pathologic diagnoses. However, this method is limited in its ability to specify cellular morphology and immunophenotype and often requires consumption of limited tissue. This study aimed to describe an optimized protocol for multiple in situ hybridization (ISH) and immunohistochemistry (IHC).
Methods
The quality of multistaining was evaluated by carefully changing each step of ISH and IHC in an angioimmunoblastic T-cell lymphoma (AITL) case on a Ventana BenchMark XT automated immunostainer. The optimized protocols were also performed using another immunostainer and in 15 cases of five Epstein-Barr virus (EBV)–associated malignancies using formalin-fixed paraffin-embedded tissue.
Results
The quality of various ISHIHC staining protocols was semi-quantitatively evaluated. The best EBV-encoded RNA (EBER)-ISH/double IHC staining quality, equivalent to single staining, was obtained using the following considerations: initial EBER-ISH application, use of protease and antigen retrieval reagent (cell conditioning 1 [CC1] treatment time was minimized due to impact on tissue quality), additional baking/ deparaffinization not needed, and reduced dilution ratio and increased reaction time for primary antibody compared with single immunostaining. Furthermore, shorter second CC1 treatment time yielded better results. Multiple staining was the best quality in another immunostainer and for different types of EBV-associated malignancies when it was performed in the same manner as for the Ventana BenchMark XT as determined for AITL.
Conclusions
EBER-ISH and double IHC could be easily used in clinical practice with currently available automated immunostainers and adjustment of reagent treatment time, dilution ratio, and antibody reaction time. -
Citations
Citations to this article as recorded by- Clinicopathologic Analysis of 34 Japanese Patients With EBV-Associated Reactive Lymphoid Hyperplasias
Yuuki Yamamoto, Akira Satou, Taishi Takahara, Daisuke Yamashita, Masafumi Seki, Akari Iwakoshi, Yusuke Ueda, Yasufumi Masaki, Kanae Yoshikawa, Hideki Murakami, Seiichi Kato, Kennosuke Karube, Shigeo Nakamura, Toyonori Tsuzuki
American Journal of Surgical Pathology.2026;[Epub] CrossRef - Ultra High-plex Spatial Proteogenomic Investigation of Giant Cell Glioblastoma Multiforme Immune Infiltrates Reveals Distinct Protein and RNA Expression Profiles
Shilah A. Bonnett, Alyssa B. Rosenbloom, Giang T. Ong, Mark Conner, Aric B.E. Rininger, Daniel Newhouse, Felicia New, Chi Q. Phan, Saskia Ilcisin, Hiromi Sato, John S. Lyssand, Gary Geiss, Joseph M. Beechem
Cancer Research Communications.2023; 3(5): 763. CrossRef - Detection of Epstein–Barr Virus in Periodontitis: A Review of Methodological Approaches
Lilit Tonoyan, Marlène Chevalier, Séverine Vincent-Bugnas, Robert Marsault, Alain Doglio
Microorganisms.2020; 9(1): 72. CrossRef
- Clinicopathologic Analysis of 34 Japanese Patients With EBV-Associated Reactive Lymphoid Hyperplasias
- An Autopsy Case of Epstein-Barr Virus–Associated Diffuse Large B-Cell Lymphoma of the Central Nervous System in an Immunocompromised Host
- Sun-Young Park, Seong Ik Kim, Hannah Kim, Yoojin Lee, Sung-Hye Park
- J Pathol Transl Med. 2018;52(1):51-55. Published online August 4, 2017
- DOI: https://doi.org/10.4132/jptm.2017.01.23
- 9,575 View
- 173 Download
- 2 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF - Lymphomas arising in the central nervous system (CNS) of immunocompromised hosts are most commonly non-Hodgkin’s lymphomas and are highly associated with Epstein-Barr virus (EBV). Here we report an autopsy case of EBV-associated CNS diffuse large B-cell lymphoma (DLBCL) in a host suffering from systemic lupus erythematosus who underwent immunosuppressive therapy. After autopsy, EBV-associated CNS DLBCL as well as pulmonary mixed aspergillosis and Pneumocystis jirovecii pneumonia were added to the cause of clinical manifestations of complicated pneumonia and cerebral hemorrhage in this immunocompromised patient. In conclusion, complex disease processes were revealed by autopsy in this case, indicating that the clinicopathological correlations observed through autopsy can improve our understanding of disease progression and contribute to the management of similar patients in the future.
-
Citations
Citations to this article as recorded by- Primary central nervous system lymphoma in neuropsychiatric systemic lupus erythematosus: case-based review
Takanori Ichikawa, Yasuhiro Shimojima, Dai Kishida, Tomoki Kaneko, Yoshiki Sekijima
Rheumatology International.2021; 41(5): 1009. CrossRef
- Primary central nervous system lymphoma in neuropsychiatric systemic lupus erythematosus: case-based review
- Epstein-Barr Virus–Associated Lymphoproliferative Disorders: Review and Update on 2016 WHO Classification
- Hyun-Jung Kim, Young Hyeh Ko, Ji Eun Kim, Seung-Sook Lee, Hyekyung Lee, Gyeongsin Park, Jin Ho Paik, Hee Jeong Cha, Yoo-Duk Choi, Jae Ho Han, Jooryung Huh
- J Pathol Transl Med. 2017;51(4):352-358. Published online June 5, 2017
- DOI: https://doi.org/10.4132/jptm.2017.03.15
- 29,377 View
- 1,121 Download
- 69 Web of Science
- 67 Crossref
-
 Abstract
Abstract
 PDF
PDF - Epstein-Barr virus (human herpesvirus-4) is very common virus that can be detected in more than 95% of the human population. Most people are asymptomatic and live their entire lives in a chronically infected state (IgG positive). However, in some populations, the Epstein-Barr virus (EBV) has been involved in the occurrence of a wide range of B-cell lymphoproliferative disorders (LPDs), including Burkitt lymphoma, classic Hodgkin’s lymphoma, and immune–deficiency associated LPDs (post-transplant and human immunodeficiency virus–associated LPDs). T-cell LPDs have been reported to be associated with EBV with a subset of peripheral T-cell lymphomas, angioimmunoblastic T-cell lymphomas, extranodal nasal natural killer/T-cell lymphomas, and other rare histotypes. This article reviews the current evidence covering EBV-associated LPDs based on the 2016 classification of the World Health Organization. These LPD entities often pose diagnostic challenges, both clinically and pathologically, so it is important to understand their unique pathophysiology for correct diagnoses and optimal management.
-
Citations
Citations to this article as recorded by- Nodal T-follicular helper cell lymphoma with hodgkin/reed-sternberg-like cells: Clinicopathologic and molecular characterization of 11 cases
Linlin Huang, Jing Li, Huawen Wang, Mei Tang, Min Jing, Xinyi Shi, Yongkun Xiao, Lu Bai, Ling Wang, Dong Liu, Tao Wu, Chao Ding, Jinglong Lv, Huami Ye, Jing Li, Jiamei Fan, Pengchun Wu, Wenbo Zhou, Xiaohui Wu, Hongwei Wang
Pathology - Research and Practice.2026; 278: 156327. CrossRef - Pathogenesis, treatment and prevention of diseases caused by Epstein–Barr virus
A. G. Rumyantsev
Pediatric Hematology/Oncology and Immunopathology.2025; 22(2): 166. CrossRef - Epstein-Barr virus–driven cardiolipin synthesis sustains metabolic remodeling during B cell transformation
Haixi You, Larissa Havey, Zhixuan Li, Yin Wang, John M. Asara, Rui Guo
Science Advances.2025;[Epub] CrossRef - Epstein-Barr virus-transformed B-cells from a hypoxia model of the germinal center requires external unsaturated fatty acids
Larissa Havey, Haixi You, Huimin Xian, John M. Asara, Rui Guo, Bill Sugden
PLOS Pathogens.2025; 21(11): e1013694. CrossRef - Case Report: Periorbital Edema as an Overlooked Presentation of Epstein-Barr Virus
Simran Ohri, Iden Amiri, Gitanjali M. Fleischman, David Fleischman
Case Reports in Ophthalmology.2025; 16(1): 834. CrossRef - Relationship between Epstein-Barr virus and inflammatory bowel disease
Su-Ying Li, Jia Jia, Lu-Zhou Xu, Kai Zheng
World Journal of Gastroenterology.2025;[Epub] CrossRef - Epstein–Barr virus‐positive monoclonal lymphoplasmacytic proliferation associated with neurosyphilis in an immunocompetent patient: A case report
Takashi Hibiya, Kiyotaka Nagahama, Yoshie Matsumoto, Kuniaki Saito, Nobuyoshi Sasaki, Keiichi Kobayashi, Akiyasu Otsu, Teppei Shimasaki, Kengo Takeuchi, Yoshiaki Shiokawa, Motoo Nagane, Junji Shibahara
Neuropathology.2024; 44(2): 104. CrossRef - Epstein-Barr virus-positive iris diffuse large B-cell lymphoma detected by metagenomic next-generation sequencing
Xiao-na Wang, Jing Hong, Yong-gen Xu, Pei Zhang, Ying-yu Li, Hong-liang Dou, Hai-ping Li
BMC Ophthalmology.2024;[Epub] CrossRef - Coinfection of EBV with other pathogens: a narrative review
Fatemeh Ebrahimi, Reyhaneh Rasizadeh, Shabnam Sharaflou, Parisa Shiri Aghbash, Ali Shamekh, Abolfazl Jafari-Sales, Hossein Bannazadeh Baghi
Frontiers in Virology.2024;[Epub] CrossRef - Pharmacological Modulation of the Crosstalk between Aberrant Janus Kinase Signaling and Epigenetic Modifiers of the Histone Deacetylase Family to Treat Cancer
Al-Hassan M. Mustafa, Oliver H. Krämer
Pharmacological Reviews.2023; 75(1): 35. CrossRef - Autophagy-associated immune dysregulation and hyperplasia in a patient with compound heterozygous mutations in ATG9A
Guowu Hu, Pia J Hauk, Nannan Zhang, Waleed Elsegeiny, Carlos M. Guardia, Amy Kullas, Kevin Crosby, Robin R. Deterding, Michaela Schedel, Paul Reynolds, Jordan K Abbott, Vijaya Knight, Stefania Pittaluga, Mark Raffeld, Sergio D. Rosenzweig, Juan S. Bonifac
Autophagy.2023; 19(2): 678. CrossRef - When to suspect inborn errors of immunity in Epstein–Barr virus–related lymphoproliferative disorders
Keith A. Sacco, Luigi D. Notarangelo, Ottavia M. Delmonte
Clinical Microbiology and Infection.2023; 29(4): 457. CrossRef - Primary head and neck cancer cell cultures are susceptible to proliferation of Epstein-Barr virus infected lymphocytes
Senyao Shao, Lars Uwe Scholtz, Sarah Gendreizig, Laura Martínez-Ruiz, Javier Florido, Germaine Escames, Matthias Schürmann, Carsten Hain, Leonie Hose, Almut Mentz, Pascal Schmidt, Menghang Wang, Peter Goon, Michael Wehmeier, Frank Brasch, Jörn Kalinowski,
BMC Cancer.2023;[Epub] CrossRef - Clinical and genetic characterization of Epstein-Barr virus–associated T/NK-cell lymphoproliferative diseases
Hui Luo, Dan Liu, Wenbing Liu, Jin Jin, Xiaoman Bi, Peiling Zhang, Jia Gu, Miao Zheng, Min Xiao, Xin Liu, Jianfeng Zhou, Qian-Fei Wang
Journal of Allergy and Clinical Immunology.2023; 151(4): 1096. CrossRef - Outcomes of programmed death protein-1 inhibitors treatment of chronic active Epstein Barr virus infection: A single center retrospective analysis
Yaxian Ma, Peiling Zhang, Yuhan Bao, Hui Luo, Jiachen Wang, Liang Huang, Miao Zheng
Frontiers in Immunology.2023;[Epub] CrossRef - Epstein–Barr virus-associated B-cell lymphoproliferative disorder meeting the definition of CAEBV B cell disease: a case report
Yaxian Ma, Yuhan Bao, Miao Zheng
BMC Infectious Diseases.2023;[Epub] CrossRef - Unpacking the CNS Manifestations of Epstein-Barr Virus: An Imaging Perspective
N. Soni, M. Ora, R. Singh, P. Mehta, A. Agarwal, G. Bathla
American Journal of Neuroradiology.2023; 44(9): 1002. CrossRef - Oncoviruses: Induction of cancer development and metastasis by increasing anoikis resistance
Zahra Sobhi Amjad, Ali Shojaeian, Javid Sadri Nahand, Mobina Bayat, Mohammad Taghizadieh, Mosayeb Rostamian, Farhad Babaei, Mohsen Moghoofei
Heliyon.2023; 9(12): e22598. CrossRef - Frequency and association of Epstein-Barr Virus genotype in rheumatoid arthritis patients of Khyber Pakhtunkhwa, Pakistan
Ayesha Munir, Suleman Khan, Sanaullah Khan, Sobia Attaullah, Mehwish Munir, Aisha Saleem, Ijaz Ali, Hideo Kato
PLOS ONE.2023; 18(12): e0295124. CrossRef - Successful treatment by using a modified SMILE regimen and autologous hematopoietic stem cell transplantation in a pediatric primary EBV-positive nodular NK/T cell lymphoma patient
Jian Li, Juxin Ye, Yongren Wang, Jun Wang, Yongjun Fang
Annals of Hematology.2022; 101(2): 433. CrossRef - Genetic errors of immunity distinguish pediatric nonmalignant lymphoproliferative disorders
Lisa R. Forbes, Olive S. Eckstein, Nitya Gulati, Erin C. Peckham-Gregory, Nmazuo W. Ozuah, Joseph Lubega, Nader K. El-Mallawany, Jennifer E. Agrusa, M. Cecilia Poli, Tiphanie P. Vogel, Natalia S. Chaimowitz, Nicholas L. Rider, Emily M. Mace, Jordan S. Ora
Journal of Allergy and Clinical Immunology.2022; 149(2): 758. CrossRef - EBV-positive B-cell ulcerative proliferation in the oral cavity associated with EBV-negative follicular lymphoma in a patient with common variable immunodeficiency: A case report and review of the literature
Waleed A. Alamoudi, Antoine Azar, Stefan K. Barta, Faizan Alawi, Takako I. Tanaka, Eric T. Stoopler, Thomas P. Sollecito
Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology.2022; 133(1): e10. CrossRef - Necrotizing Follicular Lymphoma of the Inguinal Region with Sternbergoid Cells: Clinical–Pathological Features of a Challenging Entity
Federico Scarmozzino, Marco Pizzi, Marta Sbaraglia, Luisa Santoro, Luca Frison, Silvia Nalio, Laura Bonaldi, Livio Trentin, Angelo Paolo Dei Tos
Applied Sciences.2022; 12(3): 1290. CrossRef - High percentages of peripheral blood T-cell activation in childhood Hodgkin's lymphoma are associated with inferior outcome
Fengqing Cai, Hui Gao, Zhongsheng Yu, Kun Zhu, Weizhong Gu, Xiaoping Guo, Xiaojun Xu, Hongqiang Shen, Qiang Shu
Frontiers in Medicine.2022;[Epub] CrossRef - Case Report of a Novel NFkB Mutation in a Lymphoproliferative Disorder Patient
Khashayar Danandeh, Parnian Jabbari, Elham Rayzan, Samaneh Zoghi, Sepideh Shahkarami, Raul Jimenez Heredia, Ana Krolo, Bibi Shahin Shamsian, Kaan Boztug, Nima Rezaei
Endocrine, Metabolic & Immune Disorders - Drug Targets.2022; 22(10): 1040. CrossRef - EBV-associated diseases: Current therapeutics and emerging technologies
Srishti Chakravorty, Behdad Afzali, Majid Kazemian
Frontiers in Immunology.2022;[Epub] CrossRef - Clinical features and treatment strategies for post-transplant and iatrogenic immunodeficiency-associated lymphoproliferative disorders
Akihiro Ohmoto, Shigeo Fuji
Blood Reviews.2021; 49: 100807. CrossRef - Comparative Study on Epstein-Barr Virus-Positive Mucocutaneous Ulcer and Methotrexate-Associated Lymphoproliferative Disorders Developed in the Oral Mucosa: A Case Series of 10 Patients and Literature Review
Kyoichi Obata, Tatsuo Okui, Sawako Ono, Koki Umemori, Shoji Ryumon, Kisho Ono, Mayumi Yao, Norie Yoshioka, Soichiro Ibaragi, Akira Sasaki
Diagnostics.2021; 11(8): 1375. CrossRef - Primary age‐related EBV‐associated effusion‐based lymphoma successfully treated with rituximab and thoracentesis
Justin J. Kuhlman, Muhamad Alhaj Moustafa, Alexander J. Tun, David M. Menke, Han W. Tun, Liuyan Jiang
Clinical Case Reports.2021;[Epub] CrossRef - Viral Manipulation of the Host Epigenome as a Driver of Virus-Induced Oncogenesis
Shimaa Hassan AbdelAziz Soliman, Arturo Orlacchio, Fabio Verginelli
Microorganisms.2021; 9(6): 1179. CrossRef - Spontaneous Regression of Chronic Epstein –Barr Virus Infection-Related Lymphoproliferative Disease
Bharti Kumari, Akshata Rao, Manicka Saravanan Subramanian, Aparajit Ballav Dey
Journal of the Indian Academy of Geriatrics.2021; 17(1): 40. CrossRef - The Pivotal Role of Viruses in the Pathogeny of Chronic Lymphocytic Leukemia: Monoclonal (Type 1) IgG K Cryoglobulinemia and Chronic Lymphocytic Leukemia Diagnosis in the Course of a Human Metapneumovirus Infection
Jérémy Barben, Alain Putot, Anca-Maria Mihai, Jérémie Vovelle, Patrick Manckoundia
Viruses.2021; 13(1): 115. CrossRef - B cells in multiple sclerosis — from targeted depletion to immune reconstitution therapies
Maria T. Cencioni, Miriam Mattoscio, Roberta Magliozzi, Amit Bar-Or, Paolo A. Muraro
Nature Reviews Neurology.2021; 17(7): 399. CrossRef - Development of Mast Cell and Eosinophil Hyperplasia and HLH/MAS-Like Disease in NSG-SGM3 Mice Receiving Human CD34+ Hematopoietic Stem Cells or Patient-Derived Leukemia Xenografts
Laura J. Janke, Denise M. Imai, Heather Tillman, Rosalinda Doty, Mark J. Hoenerhoff, Jiajie J. Xu, Zachary T. Freeman, Portia Allen, Natalie Wall Fowlkes, Ilaria Iacobucci, Kirsten Dickerson, Charles G. Mullighan, Peter Vogel, Jerold E. Rehg
Veterinary Pathology.2021; 58(1): 181. CrossRef - Viral coinfections in COVID‐19
Parisa S. Aghbash, Narges Eslami, Milad Shirvaliloo, Hossein B. Baghi
Journal of Medical Virology.2021; 93(9): 5310. CrossRef - Genetic predisposition to lymphomas: Overview of rare syndromes and inherited familial variants
Bartosz Szmyd, Wojciech Mlynarski, Agata Pastorczak
Mutation Research/Reviews in Mutation Research.2021; 788: 108386. CrossRef - Acute Epstein‐Barr virus associated haemophagocytosis in an Asian female: What is the diagnosis?
Soumya Ojha, Guiyi Ho, Cheryl X. Q. Lim, Siok B. Ng, Sanjay de Mel
American Journal of Hematology.2021; 96(11): 1541. CrossRef - Epstein Barr Virus: Development of Vaccines and Immune Cell Therapy for EBV-Associated Diseases
Xinle Cui, Clifford M. Snapper
Frontiers in Immunology.2021;[Epub] CrossRef - Recent Advances in Diagnosis and Therapy of Angioimmunoblastic T Cell Lymphoma
Mostafa F. Mohammed Saleh, Ahmed Kotb, Ghada E. M. Abdallah, Ibrahim N. Muhsen, Riad El Fakih, Mahmoud Aljurf
Current Oncology.2021; 28(6): 5480. CrossRef - Post-transplant lymphoproliferative disorder in adult renal transplant recipients: case series and review of literature
Dorota Kamińska, Magdalena Krajewska, Oktawia Mazanowska, Paweł Poznański, Maria Boratyńska, Marian Klinger
Central European Journal of Immunology.2021; 45(4): 498. CrossRef - Intestinal ulcers as an initial finding in EBV-associated lymphoproliferative disorder
Sizhu Wang, Yinghuan Dai, Jie Zhang, Dalian Ou, Chunhui Ouyang, Fanggen Lu
Medicine.2020; 99(3): e18764. CrossRef - Microbes as Master Immunomodulators: Immunopathology, Cancer and Personalized Immunotherapies
Joana R. Lérias, Georgia Paraschoudi, Eric de Sousa, João Martins, Carolina Condeço, Nuno Figueiredo, Carlos Carvalho, Ernest Dodoo, Mireia Castillo-Martin, Antonio Beltrán, Dário Ligeiro, Martin Rao, Alimuddin Zumla, Markus Maeurer
Frontiers in Cell and Developmental Biology.2020;[Epub] CrossRef - Epstein Barr Virus-associated Pediatric Neoplasms
Mozhgan Hashemieh, Fariba Shirvani
Archives of Pediatric Infectious Diseases.2020;[Epub] CrossRef - Novel IRF8 and PD-L1 molecular aberrations in systemic EBV-positive T-cell lymphoma of childhood
Atif Saleem, Rohan Joshi, Li Lei, Lhara Lezama, Shyam S. Raghavan, Nastaran Neishaboori, Mohana Roy, Joe Schroers-Martin, Gregory W. Charville, Christian Kunder, Brent Tan, Beth A. Martin, Yasodha Natkunam
Human Pathology: Case Reports.2020; 19: 200356. CrossRef - Fatal SARS-CoV-2 coinfection in course of EBV-associated lymphoproliferative disease
Luca Roncati, Beatrice Lusenti, Vincenzo Nasillo, Antonio Manenti
Annals of Hematology.2020; 99(8): 1945. CrossRef - Epstein-Barr Virus and the Eye
Emmett T. Cunningham, Manfred Zierhut
Ocular Immunology and Inflammation.2020; 28(4): 533. CrossRef - An atypical systemic form of chronic active EBV infection
Neha Gupta, Adam Bagg
Leukemia & Lymphoma.2020; 61(12): 3030. CrossRef - A Shared TCR Bias toward an Immunogenic EBV Epitope Dominates in HLA-B*07:02–Expressing Individuals
Louise C Rowntree, Thi H O Nguyen, Carine Farenc, Hanim Halim, Luca Hensen, Jamie Rossjohn, Tom C Kotsimbos, Anthony W Purcell, Katherine Kedzierska, Stephanie Gras, Nicole A Mifsud
The Journal of Immunology.2020; 205(6): 1524. CrossRef - Chronic active Epstein–Barr virus infection manifesting as coronary artery aneurysm and uveitis
Haijuan Xiao, Bing Hu, Rongmu Luo, Huili Hu, Junmei Zhang, Weiying Kuang, Rui Zhang, Li Li, Gang Liu
Virology Journal.2020;[Epub] CrossRef - Epstein-Barr Virus (EBV)-induced B-cell Lymphoproliferative Disorder Mimicking the Recurrence of EBV-associated Hemophagocytic Lymphohistiocytosis
Yuki Yatsushiro, Takuro Nishikawa, Aki Saito, Yozo Nakazawa, Ken-Ichi Imadome, Shunsuke Nakagawa, Yuichi Kodama, Yasuhiro Okamoto, Hirokazu Kanegane, Yoshifumi Kawano
Journal of Pediatric Hematology/Oncology.2019; 41(1): e44. CrossRef - Epstein-Barr Virus (EBV)-Related Lymphoproliferative Disorders in Ataxia Telangiectasia: Does ATM Regulate EBV Life Cycle?
Moussab Tatfi, Olivier Hermine, Felipe Suarez
Frontiers in Immunology.2019;[Epub] CrossRef - The factors associated with the early diagnosis of nasal NK/T-cell lymphoma with prominent ocular symptoms and general nasal NKTL
Zhen zhen Hu, Ying Wang
American Journal of Otolaryngology.2019; 40(3): 353. CrossRef - Unusual lymphoid malignancy and treatment response in two children with Down syndrome
Ashley Geerlinks, Jennifer Keis, Bo Ngan, Amer Shammas, Reza Vali, Johann Hitzler
Pediatric Blood & Cancer.2019;[Epub] CrossRef - Extreme Peripheral Blood Plasmacytosis Mimicking Plasma Cell Leukemia as a Presenting Feature of Angioimmunoblastic T-Cell Lymphoma (AITL)
Kelsey Sokol, Saritha Kartan, William T. Johnson, Onder Alpdogan, Neda Nikbakht, Bradley M. Haverkos, Jerald Gong, Pierluigi Porcu
Frontiers in Oncology.2019;[Epub] CrossRef - High-Throughput Sequence Analysis of Peripheral T-Cell Lymphomas Indicates Subtype-Specific Viral Gene Expression Patterns and Immune Cell Microenvironments
Hani Nakhoul, Zhen Lin, Xia Wang, Claire Roberts, Yan Dong, Erik Flemington, Blossom Damania
mSphere.2019;[Epub] CrossRef - Quercetin Interrupts the Positive Feedback Loop Between STAT3 and IL-6, Promotes Autophagy, and Reduces ROS, Preventing EBV-Driven B Cell Immortalization
Marisa Granato, Maria Saveria Gilardini Montani, Claudia Zompetta, Roberta Santarelli, Roberta Gonnella, Maria Anele Romeo, Gabriella D’Orazi, Alberto Faggioni, Mara Cirone
Biomolecules.2019; 9(9): 482. CrossRef - Diffuse Large B-Cell Lymphoma Arising within Ileal Neobladder: An Expanding Spectrum of Diffuse Large B-Cell Lymphoma Associated with Chronic Inflammation
Hyekyung Lee, Hyunbin Shin, Nae Yu Kim, Hyun Sik Park, Jinsung Park
Cancer Research and Treatment.2019; 51(4): 1666. CrossRef - EBV-associated lymphoproliferative disorder involving the gastrointestinal tract which mimic IBD in immunocompetent patients: case reports and literature review
Yanhua Zhou, Yanlin Zhang, Haiying Zhao, Xuan Cui, Yongqiu Wei, Yongdong Wu, Shutian Zhang, Ye Zong
International Journal of Colorectal Disease.2019; 34(11): 1989. CrossRef - Mechanistic Insights into Chemoresistance Mediated by Oncogenic Viruses in Lymphomas
Jungang Chen, Samantha Kendrick, Zhiqiang Qin
Viruses.2019; 11(12): 1161. CrossRef - Rapidly Fatal Encephalitis Associated with Atypical Lymphoid Proliferations of the Basal Ganglia Subsequent to Aneurysmal Subarachnoid Hemorrhage
Ayesha Kar, Evin L. Guilliams, Joshua A. Cuoco, Eric A. Marvin
Clinics and Practice.2019; 9(4): 1187. CrossRef - Clinicopathologic features of adult EBV-associated B-cell lymphoproliferative disease
Sonja Wörner, Hans-Konrad Mueller-Hermelink, Hans-Ullrich Voelker
Pathology - Research and Practice.2018; 214(2): 207. CrossRef - Primary Intestinal Epstein–Barr Virus-associated Natural Killer/T-cell Lymphoproliferative Disorder: A Disease Mimicking Inflammatory Bowel Disease
Zhujun Wang, Wenyan Zhang, Chengxin Luo, Min Zhu, Yu Zhen, Jingxi Mu, Yan Zhang, Renwei Hu, Yufang Wang, Zhonghui Wen, Qin Ouyang, Shuyuan Xiao, Hu Zhang
Journal of Crohn's and Colitis.2018; 12(8): 896. CrossRef - Downregulation of CD5 and dysregulated CD8+ T‐cell activation
Taizo Wada
Pediatrics International.2018; 60(9): 776. CrossRef - Chronic active Epstein-Barr virus infection of T-cell type, systemic form in an African migrant: case report and review of the literature on diagnostics standards and therapeutic options
Maxi Wass, Marcus Bauer, Roald Pfannes, Kerstin Lorenz, Andreas Odparlik, Lutz P Müller, Claudia Wickenhauser
BMC Cancer.2018;[Epub] CrossRef - Aggressive B-cell lymphomas in patients with myelofibrosis receiving JAK1/2 inhibitor therapy
Edit Porpaczy, Sabrina Tripolt, Andrea Hoelbl-Kovacic, Bettina Gisslinger, Zsuzsanna Bago-Horvath, Emilio Casanova-Hevia, Emmanuelle Clappier, Thomas Decker, Sabine Fajmann, Daniela A. Fux, Georg Greiner, Sinan Gueltekin, Gerwin Heller, Harald Herkner, Gr
Blood.2018; 132(7): 694. CrossRef - Gammaherpesviral infections in patients with immunological disorders
Anna Żuk-Wasek, Maciej Przybylski, Natalia Żeber, Grażyna Młynarczyk, Tomasz Dzieciątkowski
Postępy Mikrobiologii - Advancements of Microbiology.2018; 57(2): 145. CrossRef - COMPARATIVE ANALYSIS OF SEROLOGICAL MARKERS OF HERPES VIRUSES AND QUANTITATIVE IMMUNOGLOBULINOPATHIES IN PRIMARY PATIENTS WITH ANGIOIMMUNOBLASTIC T-CELL LYMPHOMA
N. G. Chernova, D. S. Tihomirov, N. P. Soboleva, S. A. Mariina, Y. V. Sidorova, M. N. Sinitsyna, V. N. Dvirnyk, S. M. Kulikov, T. A. Tupoleva, E. E. Zvonkov
Problems of Virology.2018; 63(4): 171. CrossRef
- Nodal T-follicular helper cell lymphoma with hodgkin/reed-sternberg-like cells: Clinicopathologic and molecular characterization of 11 cases
- CD30-Positive T-Cell Lymphoproliferative Disease of the Oral Mucosa in Children: A Manifestation of Epstein-Barr Virus-Associated T-Lymphoproliferative Disorder
- Mineui Hong, Young Hyeh Ko
- J Pathol Transl Med. 2015;49(6):525-530. Published online September 30, 2015
- DOI: https://doi.org/10.4132/jptm.2015.07.13
- 11,944 View
- 114 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF - Eosinophilic ulcer of the oral mucosa (EUOM) is a very rare, benign, self-limiting ulcerative lesion of the oral cavity of unknown pathogenesis, and belongs to the same spectrum of CD30+ T-cell lymphoproliferative disease (LPD) of the oral mucosa. The etiology and pathogenesis of the disease are unknown. We report two cases in children who were initially diagnosed with EUOM and CD30+ T-cell LPD, respectively. However, retrospective analysis revealed that a majority of infiltrated atypical T cells were positive for Epstein-Barr virus (EBV). The present cases suggest that the pathogenesis and etiology of EUOM or CD30+ T-cell LPD occurring in children are different from those in adults. EUOM or CD30+ T-cell LPD in children is a manifestation of EBV-positive T-cell LPD, and should therefore be distinguished from the disease in adults.
-
Citations
Citations to this article as recorded by- Pediatric oral Epstein-Barr virus associated self-remitting CD30+ lymphoproliferative disorder: A distinct entity
Ziv Schwartz, Robert B. Bowe, Morton Coleman, Cynthia M. Magro
Annals of Diagnostic Pathology.2018; 37: 57. CrossRef
- Pediatric oral Epstein-Barr virus associated self-remitting CD30+ lymphoproliferative disorder: A distinct entity
- Human Herpesvirus 8-Negative and Epstein-Barr Virus-Positive Effusion-Based Lymphoma in a Patient with Human Immunodeficiency Virus
- Jung-Woo Choi, Younghye Kim, Ju-Han Lee, Young-Sik Kim
- J Pathol Transl Med. 2015;49(5):409-412. Published online June 17, 2015
- DOI: https://doi.org/10.4132/jptm.2015.06.03
- 9,716 View
- 65 Download
- 3 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF - A 39-year-old man infected with human immunodeficiency virus (HIV) was admitted to our hospital because of sudden onset of chest pain. Chest radiography revealed pneumothorax of the right lung. Computed tomographic scans disclosed a 5.8-cm-sized emphysematous bulla in the right middle lobe of the lung. Histologically, the wedge-resected lung showed medium to large atypical cells within the bullous cavity of the Pneumocystis jirovecii pneumonia, without solid mass formation. These atypical cells were confirmed to be large B-cell lymphoma, Epstein-Barr virus–positive and human herpesvirus 8–negative. Therefore, this case was not diagnosed as primary effusion lymphoma, but effusion-based lymphoma arising in an emphysematous cavity of an HIV-infected patient. This type of effusion-based lymphoma has never been reported, and, although rare, it should be noted in order to clinically diagnose this lymphoma.
-
Citations
Citations to this article as recorded by- Primary Effusion Lymphoma: A Timely Review on the Association with HIV, HHV8, and EBV
Chih-Yi Liu, Bo-Jung Chen, Shih-Sung Chuang
Diagnostics.2022; 12(3): 713. CrossRef - Human herpesvirus 8-negative effusion-based large B-cell lymphoma: a distinct entity with unique clinicopathologic characteristics
Savanah D. Gisriel, Ji Yuan, Ryan C. Braunberger, Danielle L.V. Maracaja, Xueyan Chen, Xiaojun Wu, Jenna McCracken, Mingyi Chen, Yi Xie, Laura E. Brown, Peng Li, Yi Zhou, Tarsheen Sethi, Austin McHenry, Ronald G. Hauser, Nathan Paulson, Haiming Tang, Eric
Modern Pathology.2022; 35(10): 1411. CrossRef - Age and CD20 Expression Are Significant Prognostic Factors in Human Herpes Virus-8-negative Effusion-based Lymphoma
Tomomi Kubota, Yosuke Sasaki, Eisuke Shiozawa, Masafumi Takimoto, Tsunekazu Hishima, Ja-Mun Chong
American Journal of Surgical Pathology.2018; 42(12): 1607. CrossRef
- Primary Effusion Lymphoma: A Timely Review on the Association with HIV, HHV8, and EBV
- Follicular Dendritic Cell Sarcoma of the Inflammatory Pseudotumor-like Variant Presenting as a Colonic Polyp
- Shien-Tung Pan, Chih-Yuan Cheng, Nie-Sue Lee, Peir-In Liang, Shih-Sung Chuang
- Korean J Pathol. 2014;48(2):140-145. Published online April 28, 2014
- DOI: https://doi.org/10.4132/KoreanJPathol.2014.48.2.140
- 11,500 View
- 105 Download
- 34 Crossref
-
 Abstract
Abstract
 PDF
PDF Follicular dendritic cell (FDC) sarcoma is rare and is classified either as conventional type or inflammatory pseudotumor (IPT)-like variant. Extranodal presentation is uncommon and nearly all gastrointestinal FDC tumors are of the conventional type. IPT-like variant tumors occur almost exclusively in the liver and spleen and are consistently associated with Epstein-Barr virus (EBV). Here we report the case of a 78-year-old woman with an IPT-like FDC sarcoma presenting as a pedunculated colonic polyp. Histologically, scanty atypical ovoid to spindle cells were mixed with a background of florid lymphoplasmacytic infiltrate, which led to an initial misdiagnosis of pseudolymphoma. These atypical cells expressed CD21, CD23, CD35, and D2-40, and were positive for EBV by
in situ hybridization, confirming the diagnosis. The patient was free of disease five months after polypectomy without adjuvant therapy. Although extremely rare, the differential diagnosis for colonic polyp should include FDC sarcoma to avoid an erroneous diagnosis. A review of the 24 cases of IPT-like FDC sarcoma reported in the literature reveal that this tumor occurs predominantly in females with a predilection for liver and spleen, and has a strong association with EBV.-
Citations
Citations to this article as recorded by- The fifth edition of the WHO classification of mature T cell, NK cell and stroma-derived neoplasms
Ayoma D Attygalle, Kennosuke Karube, Yoon Kyung Jeon, Wah Cheuk, Govind Bhagat, John K C Chan, Kikkeri N Naresh
Journal of Clinical Pathology.2025; 78(4): 217. CrossRef - Genomic and Transcriptomic Landscape of Epstein-Barr Virus-Positive Inflammatory Follicular Dendritic Cell Sarcoma: A Multicenter Study
Yan Li, Ze-Lin Weng, Han-Xiao Fei, Hai-Feng Li, Yi-Na Liu, Le-Le Zhang, Qiong Zhang, Xin Weng, Yuan-Yuan Wang, Wen-Yong Huang, Zhi-Xing Cao, Kai-Yan Yang, Xi-Liang Chen, Jie Gao, Wen-Sheng Yang, Fang Liu, Juan-Juan Yong, Jing-Ping Yun, Hua Zhang, Yu-Hua H
Modern Pathology.2025; 38(10): 100864. CrossRef - What is new in the 5th edition of the World Health Organization classification of mature B and T/NK cell tumors and stromal neoplasms?
Ayoma D. Attygalle, John K. C. Chan, Sarah E. Coupland, Ming-Qing Du, Judith A. Ferry, Daphne de Jong, Dita Gratzinger, Megan S. Lim, Alina Nicolae, German Ott, Andreas Rosenwald, Anna Schuh, Reiner Siebert
Journal of Hematopathology.2024; 17(2): 71. CrossRef - Pathologic characteristics of histiocytic and dendritic cell neoplasms
Sun Och Yoon
Blood Research.2024;[Epub] CrossRef - Epstein-barr virus (EBV)-positive inflammatory pseudotumor-like follicular dendritic cell sarcoma (IPT-like FDCS) presenting as thrombocytopenia: A case report and literature review
Jiawei Jin, Xiaolong Zhu, Yi Wan, Yang Shi
Heliyon.2024; 10(12): e32997. CrossRef - EBV-positive inflammatory follicular dendritic cell sarcoma of the colon with clonal immunoglobulin gene rearrangement: A case report and literature review
Xia Xu, Xiuzhen Li, Qun Deng, Kaihang Yu, Jinfan Li
Heliyon.2024; 10(11): e31947. CrossRef - Challenges in the Diagnosis of Epstein-Barr Virus-positive Inflammatory Follicular Dendritic Cell Sarcoma
Yan Li, Xia Yang, Lili Tao, Weimei Zeng, Min Zuo, Shuo Li, Liyan Wu, Yanshong Lin, Ziying Zhang, Jingping Yun, Yuhua Huang
American Journal of Surgical Pathology.2023; 47(4): 476. CrossRef - Epstein-Barr Virus-Positive Inflammatory Follicular Dendritic Cell Sarcoma Presenting as a Colonic Polyp: Report of a Case with a Literature Review
Jiahui Hu, Dongdong Huang, Chengfu Xu, Yi Chen, Han Ma, Zhe Shen
Medicina.2023; 59(7): 1341. CrossRef - A Clinicopathology Review and Update of Epstein–Barr Virus-Associated Mesenchymal Tumors
Oswald Zhao Jian Lee, Noorjehan Omar, Joshua K. Tay, Victor Kwan Min Lee
Cancers.2023; 15(23): 5563. CrossRef - Granulomatous splenic mass with necrosis revealing an EBV-positive inflammatory follicular dendritic cell sarcoma
Irena Antonia Ungureanu, Renato Micelli Lupinacci, Marie Parrens, Jean-François Emile
Journal of Surgical Case Reports.2022;[Epub] CrossRef - Case report: Hepatic inflammatory pseudotumor-like follicular dendritic cell sarcoma: A rare case and minireview of the literature
Fan Ding, Chao Wang, Chi Xu, Hui Tang
Frontiers in Medicine.2022;[Epub] CrossRef - Follicular dendritic cell sarcoma of gastrointestinal tract with two emerging distinct subtypes: a case report and systemic review
Hongxing Gui, Jigisha Chaudhari, Rifat Mannan
Diagnostic Pathology.2022;[Epub] CrossRef - Surgical treatment of liver inflammatory pseudotumor-like follicular dendritic cell sarcoma: A case report
Li-Yue Fu, Jiu-Liang Jiang, Meng Liu, Jun-Jun Li, Kai-Ping Liu, Hai-Tao Zhu
World Journal of Gastrointestinal Oncology.2022; 14(11): 2288. CrossRef - Inflammatory pseudotumor-like follicular/fibroblastic dendritic cell sarcoma: focus on immunohistochemical profile and association with Epstein-Barr virus
Francesca Pagliuca, Andrea Ronchi, Annamaria Auricchio, Eva Lieto, Renato Franco
Infectious Agents and Cancer.2022;[Epub] CrossRef - Recent Advances in Digestive Tract Tumors: Updates From the 5th Edition of the World Health Organization “Blue Book”
Raul S. Gonzalez, Anwar Raza, Robert Propst, Oyedele Adeyi, Justin Bateman, Sabrina C. Sopha, Janet Shaw, Aaron Auerbach
Archives of Pathology & Laboratory Medicine.2021; 145(5): 607. CrossRef - Hepatic inflammatory pseudotumor-like follicular dendritic cell tumor: a case report
Ana Daniela Pascariu, Andreea Ioana Neagu, Andrei Valentin Neagu, Alexandru Băjenaru, Cezar Iulian Bețianu
Journal of Medical Case Reports.2021;[Epub] CrossRef - Inflammatory pseudotumor-like follicular dendritic cell sarcoma: Literature review of 67 cases
Hao Wu, Peng Liu, Xiao-Ran Xie, Jing-Shu Chi, Huan Li, Can-Xia Xu
World Journal of Meta-Analysis.2021; 9(1): 1. CrossRef - New Clinicopathologic Scenarios of EBV+ Inflammatory Follicular Dendritic Cell Sarcoma
Xiang-Nan Jiang, Yan Zhang, Tian Xue, Jie-Yu Chen, Alex C.L. Chan, Wah Cheuk, John K.C. Chan, Xiao-Qiu Li
American Journal of Surgical Pathology.2021; 45(6): 765. CrossRef - Select Epstein-Barr Virus–Associated Digestive Tract Lesions for the Practicing Pathologist
Zainab I. Alruwaii, Elizabeth A. Montgomery
Archives of Pathology & Laboratory Medicine.2021; 145(5): 562. CrossRef - Overview of Gastrointestinal Lymphoproliferative disorders✰
Aaron Auerbach, Nadine S. Aguilera
Seminars in Diagnostic Pathology.2021; 38(4): 1. CrossRef - Follicular dendritic cell sarcoma
Fabio Facchetti, Matteo Simbeni, Luisa Lorenzi
Pathologica.2021; 113(5): 316. CrossRef - Hepatic inflammatory pseudotumor-like follicular dendritic cell tumor with hepatic lymphoma history
Jiang Li, Hai-su Tao, Dong Chen, Zhi-yong Huang, Er-lei Zhang
Medicine.2021; 100(39): e27392. CrossRef - Clinicopathological characteristics of extranodal follicular dendritic cell sarcoma: A report of two cases
Xing Zhao, Dayong Sun, Gang Zhang
Oncology Letters.2021;[Epub] CrossRef - Inflammatory pseudotumour-like follicular dendritic cell tumour of the colon with plasmacytosis mimicking EBV-positive lymphoproliferative disorder
Ying-Ren Chen, Chi-Lin Lee, Yen-Chien Lee, Kung-Chao Chang
Pathology.2020; 52(4): 484. CrossRef - Beware the inflammatory cell-rich colonic polyp: a rare case of EBV-positive inflammatory pseudotumour-like follicular dendritic cell sarcoma with increased IgG4-positive plasma cells
Lynne Goh, Nan Zun Teo, Lai Mun Wang
Pathology.2020; 52(6): 713. CrossRef - Epstein–Barr virus‐positive inflammatory follicular dendritic cell sarcoma presenting as a solitary colonic mass: two rare cases and a literature review
Xiaokang Ke, Huihua He, Qingping Zhang, Jingping Yuan, Qilin Ao
Histopathology.2020; 77(5): 832. CrossRef - Inflammatory pseudotumor-like follicular dendritic cell sarcoma: A brief report of two cases
Bi-Xi Zhang, Zhi-Hong Chen, Yu Liu, Yuan-Jun Zeng, Yan-Chun Li
World Journal of Gastrointestinal Oncology.2019; 11(12): 1231. CrossRef - Epstein-Barr virus (EBV)–associated lymphoid proliferations, a 2018 update
Sherif A. Rezk, Xiaohui Zhao, Lawrence M. Weiss
Human Pathology.2018; 79: 18. CrossRef - A Rare Case of Epstein-Barr Virus Negative Inflammatory Pseudotumor-like Follicular Dendritic Cell Sarcoma Presenting as a Solitary Colonic Mass in a 53-Year-Old Woman; Case Report and Review of Literature
Rossana Kazemimood, Farid Saei Hamedani, Asma Sharif, Sujata Gaitonde, Elizabeth Wiley, Pier Cristoforo Giulianotti, John Vincent Groth
Applied Immunohistochemistry & Molecular Morphology.2017; 25(5): e30. CrossRef - A Case of Inflammatory Pseudotumor-like Follicular Dendritic Cell Sarcoma of the Lymph Node in the Small Bowel Mesentery Accompanied by Myasthenia Gravis
Daichi KITAGUCHI, Katsuji HISAKURA, Taiki SATO, Masanao KURATA, Tatsuya ODA, Nobuhiro OHKOHCHI
Nihon Rinsho Geka Gakkai Zasshi (Journal of Japan Surgical Association).2017; 78(3): 527. CrossRef - Clinicopathological features of inflammatory pseudotumour‐like follicular dendritic cell tumour of the abdomen
Yanyang Chen, Huijuan Shi, Hui Li, Tiantian Zhen, Anjia Han
Histopathology.2016; 68(6): 858. CrossRef - A Rare Case of Follicular Dendritic Cell Sarcoma with Pseudochylous Effusion and Review of Literature From India
Kamal Kant Sahu, Gaurav Prakash, Sandeep Rao, Amanjit Bal, Pankaj Malhotra, Jasmina Ahluwalia, Rakesh K. Vashistha
Indian Journal of Hematology and Blood Transfusion.2015; 31(2): 307. CrossRef - Epstein-Barr virus–associated inflammatory pseudotumor presenting as a colonic mass
Shunyou Gong, Iwona Auer, Rajan Duggal, Stefania Pittaluga, Mark Raffeld, Elaine S. Jaffe
Human Pathology.2015; 46(12): 1956. CrossRef - Response of follicular dendritic cell sarcoma to gemcitabine and docetaxel: report of two cases and literature review
Robert M Conry
Clinical Sarcoma Research.2014;[Epub] CrossRef
- The fifth edition of the WHO classification of mature T cell, NK cell and stroma-derived neoplasms
- EBV-Positive T/NK-Cell Lymphoproliferative Disease of Childhood
- Mineui Hong, Young Hyeh Ko, Keon Hee Yoo, Hong Hoe Koo, Seok Jin Kim, Won Seog Kim, Heejung Park
- Korean J Pathol. 2013;47(2):137-147. Published online April 24, 2013
- DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.2.137
- 16,275 View
- 122 Download
- 31 Crossref
-
 Abstract
Abstract
 PDF
PDF Background Epstein-Barr virus (EBV)-associated hemophagocytic lymphohistiocytosis (HLH), EBV-positive systemic T-cell lymphoproliferative disease (STLPD) of childhood, and chronic active EBV (CAEBV) infection may develop after primary EBV infection. This study reviewed the clinicopathological spectrum of EBV-associated T- and natural killer (NK)-cell LPD, including STLPD and CAEBV infection, with an analysis of T-cell clonality.
Methods Clinicopathological features of seven patients with EBV-associated HLH or STLPD and 12 patients with CAEBV infection were reviewed. Immunohistochemical staining and a T-cell receptor (TCR) gene rearrangement study were performed.
Results STLPD and EBV-positive HLH showed significantly overlapping clinicopathological findings. One patient with STLPD and one patient with EBV-positive HLH demonstrated moderate to severe atypia of the infiltrating lymphocytes, whereas the remaining patients lacked significant atypia. Twelve patients had CAEBV infection, four of whom suffered mosquito-bite hypersensitivity, five showed NK lymphocytosis, and one suffered hydroa vacciniforme. Infiltrating lymphocytes were predominantly small and devoid of atypia. Hemophagocytic histiocytosis was found in seven of 11 patients. Monoclonality was detected in three (50%) of the six patients with successful TCR gene analysis.
Conclusions EBV-positive HLH and STLPD share similar clinicopathological findings and may constitute a continuous spectrum of acute EBV-associated T- or NK-cell proliferative disorders. The distinction of EBV-positive T-cell LPD from EBV-positive HLH may be difficult during routine diagnoses because of the technical limitations of clonality assessment.
-
Citations
Citations to this article as recorded by- Histopathological characteristics of Epstein-Barr virus (EBV)–associated encephalitis and colitis in chronic active EBV infection
Betty A Kasimo, James J Yahaya, Sun Och Yoon, Se Hoon Kim, Minsun Jung
Journal of Pathology and Translational Medicine.2025; 59(3): 188. CrossRef - Die fünfte Auflage der WHO‐Klassifikation – Was ist neu für kutane Lymphome?
Susanne Melchers, Jana D. Albrecht, Werner Kempf, Jan P. Nicolay
JDDG: Journal der Deutschen Dermatologischen Gesellschaft.2024; 22(9): 1254. CrossRef - Fifth Edition of the World Health Organization Classification of Tumors of the Hematopoietic and Lymphoid Tissues: Mature T-Cell, NK-Cell, and Stroma-Derived Neoplasms of Lymphoid Tissues
Roberto N. Miranda, Catalina Amador, John K.C. Chan, Joan Guitart, Karen L. Rech, L. Jeffrey Medeiros, Kikkeri N. Naresh
Modern Pathology.2024; 37(8): 100512. CrossRef - Clinical epidemiology of Epstein-Barr virus-associated Lymphoproliferative Disorders (EBV-LPDs) in hospitalized children: A six-year multi-institutional study in China
Dilara Dilmurat, Xinyu Wang, Liwei Gao, Jiao Tian, Junhong Ai, Linlin Zhang, Mengjia Liu, Guoshuang Feng, Yueping Zeng, Ran Wang, Zhengde Xie
Italian Journal of Pediatrics.2024;[Epub] CrossRef - The fifth edition of the WHO‐Classification – what is new for cutaneous lymphomas?
Susanne Melchers, Jana D. Albrecht, Werner Kempf, Jan P. Nicolay
JDDG: Journal der Deutschen Dermatologischen Gesellschaft.2024; 22(9): 1254. CrossRef - An update on Epstein-Barr virus–and human T-lymphotropic virus type-1–induced cutaneous manifestations. CME Part II
Alejandro A. Gru, Jose A. Plaza, Jose A. Sanches, Denis Miyashiro, Omar P. Sangueza, Francisco Bravo Puccio, Sonia Toussaint, J. Martin Sangueza
Journal of the American Academy of Dermatology.2023; 88(5): 983. CrossRef - The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms
Rita Alaggio, Catalina Amador, Ioannis Anagnostopoulos, Ayoma D. Attygalle, Iguaracyra Barreto de Oliveira Araujo, Emilio Berti, Govind Bhagat, Anita Maria Borges, Daniel Boyer, Mariarita Calaminici, Amy Chadburn, John K. C. Chan, Wah Cheuk, Wee-Joo Chng,
Leukemia.2022; 36(7): 1720. CrossRef - Chronic active Epstein–Barr virus enteritis: A literature review
Yang Shen, Yu Fang Wang
Journal of Digestive Diseases.2022; 23(5-6): 248. CrossRef - EBV-Associated Lymphoproliferative Disorders
Young Hyeh Ko
Clinical Pediatric Hematology-Oncology.2021; 28(1): 14. CrossRef - Clinicopathologic findings of chronic active Epstein–Barr virus infection in adults: A single-center retrospective study in China
Jing Lin, Haicong Wu, Lei Gu, Xia Wu, Miaofang Su, Haiyan Lin, Bang Liu, Jiaolong Zheng, Xuan Mei, Dongliang Li
Clinical and Experimental Medicine.2021; 21(3): 369. CrossRef - Outcome of L-DEP regimen for treatment of pediatric chronic active Epstein–Barr virus infection
Honghao Ma, Liping Zhang, Ang Wei, Jun Yang, Dong Wang, Qing Zhang, Yunze Zhao, Sitong Chen, Hongyun Lian, Li Zhang, Chunju Zhou, Maoquan Qin, Zhigang Li, Tianyou Wang, Rui Zhang
Orphanet Journal of Rare Diseases.2021;[Epub] CrossRef - Epstein-Barr virus NK and T cell lymphoproliferative disease: report of a 2018 international meeting
Jeffrey I. Cohen, Keiji Iwatsuki, Young-Hyeh Ko, Hiroshi Kimura, Irini Manoli, Koichi Ohshima, Stefania Pittaluga, Leticia Quintanilla-Martinez, Elaine S. Jaffe
Leukemia & Lymphoma.2020; 61(4): 808. CrossRef - EBV-positive T/NK-associated lymphoproliferative disorders of childhood: A complete autopsy report
JonathanY Keow, WilliamM Stecho, AaronR Haig, NikhilA Sangle
Indian Journal of Pathology and Microbiology.2020; 63(1): 78. CrossRef - Chronic active Epstein‐Barr virus infection: A heterogeneous entity requiring a high index of suspicion for diagnosis
Sarah L. Ondrejka, Eric D. Hsi
International Journal of Laboratory Hematology.2020; 42(S1): 99. CrossRef - Epstein-Barr Virus-Associated T and NK-Cell Lymphoproliferative Diseases
Wook Youn Kim, Ivonne A. Montes-Mojarro, Falko Fend, Leticia Quintanilla-Martinez
Frontiers in Pediatrics.2019;[Epub] CrossRef - A clinicopathologic study of the spectrum of systemic forms of EBV‐associated T‐cell lymphoproliferative disorders of childhood: A single tertiary care pediatric institution experience in North America
Amy M. Coffey, Annisa Lewis, Andrea N. Marcogliese, M. Tarek Elghetany, Jyotinder N. Punia, Chung‐Che Chang, Carl E. Allen, Kenneth L. McClain, Amos S. Gaikwad, Nader Kim El‐Mallawany, Choladda V. Curry
Pediatric Blood & Cancer.2019;[Epub] CrossRef - Unusual lymphoid malignancy and treatment response in two children with Down syndrome
Ashley Geerlinks, Jennifer Keis, Bo Ngan, Amer Shammas, Reza Vali, Johann Hitzler
Pediatric Blood & Cancer.2019;[Epub] CrossRef - EBV-Positive Lymphoproliferations of B- T- and NK-Cell Derivation in Non-Immunocompromised Hosts
Stefan Dojcinov, Falko Fend, Leticia Quintanilla-Martinez
Pathogens.2018; 7(1): 28. CrossRef - Cutaneous Hematolymphoid and Histiocytic Proliferations in Children
Alejandro A Gru, Louis P Dehner
Pediatric and Developmental Pathology.2018; 21(2): 208. CrossRef - Clinicopathological categorization of Epstein–Barr virus-positive T/NK-cell lymphoproliferative disease: an analysis of 42 cases with an emphasis on prognostic implications
Jin Ho Paik, Ji-Young Choe, Hyojin Kim, Jeong-Ok Lee, Hyoung Jin Kang, Hee Young Shin, Dong Soon Lee, Dae Seog Heo, Chul-Woo Kim, Kwang-Hyun Cho, Tae Min Kim, Yoon Kyung Jeon
Leukemia & Lymphoma.2017; 58(1): 53. CrossRef - Cutaneous EBV-related lymphoproliferative disorders
Alejandro A. Gru, Elaine S. Jaffe
Seminars in Diagnostic Pathology.2017; 34(1): 60. CrossRef - T- and NK-Cell Lymphomas and Systemic Lymphoproliferative Disorders and the Immunodeficiency Setting
Dita Gratzinger, Daphne de Jong, Elaine S. Jaffe, Amy Chadburn, John K. C. Chan, John R. Goodlad, Jonathan Said, Yasodha Natkunam
American Journal of Clinical Pathology.2017; 147(2): 188. CrossRef - Systemic Epstein-Barr Virus-positive T-Cell Lymphoproliferative Disease of Childhood With Good Response to Steroid Therapy
Do-Hoon Kim, Myungshin Kim, Yonggoo Kim, Kyungja Han, Eunhee Han, Jae Wook Lee, Nack-Gyun Chung, Bin Cho
Journal of Pediatric Hematology/Oncology.2017; 39(8): e497. CrossRef - Recent advances in the risk factors, diagnosis and management of Epstein-Barr virus post-transplant lymphoproliferative disease
Paibel Aguayo-Hiraldo, Reuben Arasaratnam, Rayne H. Rouce
Boletín Médico del Hospital Infantil de México.2016; 73(1): 31. CrossRef - Severe Epstein–Barr virus infection in primary immunodeficiency and the normal host
Austen J. J. Worth, Charlotte J. Houldcroft, Claire Booth
British Journal of Haematology.2016; 175(4): 559. CrossRef - Recent advances in the risk factors, diagnosis and management of Epstein-Barr virus post-transplant lymphoproliferative disease
Paibel Aguayo-Hiraldo, Reuben Arasaratnam, Rayne H. Rouce
Boletín Médico Del Hospital Infantil de México (English Edition).2016; 73(1): 31. CrossRef - Epstein-Barr Virus–Associated Lymphomas
Ewelina Grywalska, Jacek Rolinski
Seminars in Oncology.2015; 42(2): 291. CrossRef - Epstein–Barr virus-associated T/natural killer-cell lymphoproliferative disorder in children and young adults has similar molecular signature to extranodal nasal natural killer/T-cell lymphoma but shows distinctive stem cell-like phenotype
Siok-Bian Ng, Koichi Ohshima, Viknesvaran Selvarajan, Gaofeng Huang, Shoa-Nian Choo, Hiroaki Miyoshi, Norio Shimizu, Renji Reghunathan, Hsin-Chieh Chua, Allen Eng-Juh Yeoh, Thuan-Chong Quah, Liang-Piu Koh, Poh-Lin Tan, Wee-Joo Chng
Leukemia & Lymphoma.2015; 56(8): 2408. CrossRef - An uncommon presentation of EBV-driven HLH. Primary or secondary? An ongoing dilemma
Tânia Serrão, Alexandra Dias, Pedro Nunes, António Figueiredo
BMJ Case Reports.2015; 2015: bcr2015209615. CrossRef - Hemophagocytic syndromes — An update
Gritta E. Janka, Kai Lehmberg
Blood Reviews.2014; 28(4): 135. CrossRef - Epstein–Barr virus‐associated T/natural killer‐cell lymphoproliferative disorders
Sanghui Park, Young H. Ko
The Journal of Dermatology.2014; 41(1): 29. CrossRef
- Histopathological characteristics of Epstein-Barr virus (EBV)–associated encephalitis and colitis in chronic active EBV infection
- Genetic Analysis of Epstein-Barr Virus Latent Membrane Protein 1 and Immunohistochemical Expression of Transforming Growth Factor (TGF)-beta1, TGF-betaRII, p21, p16, E2F1, Thymidylate Synthase, and NF-kappaB in Epstein-Barr Virus Encoded RNA-positive Gastric Adenocarcinoma
- Mee Yon Cho, Minseob Eom, Kwang Hwa Park, Mee Dong Kim, Seung Hoon Sung, Myoung Soo Kim, Dae Sung Kim, Sun Ju Choi
- Korean J Pathol. 2006;40(3):176-184.
- 2,098 View
- 22 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
:Although clinicopathologic differences have been described between Epstein-Barr virus (EBV)-positive and negative gastric adenocarcinomas, the pathogenetic basis for these differences remains unclear. In this study, efforts were made to confirm that expression of EBV-latent membrane protein (LMP1) and immunohistochemical characteristics of EBVpositive gastric adenocarcinomas.
METHODS
We investigated genomic deletion, and RNA & protein expression of the EBV-LMP1, as well as immunohistochemical protein expression of transforming growth factor (TGF)-beta1, TGF-bata RII, p21, p16, E2F1, thymidylate synthase, and NF-kappaB in relation to EBV positive gastric adenocarcinoma.
RESULTS
A total of 38 Epstein-Barr Virus Encoded RNA-positive and 80 negative gastric carcinomas were examined. A 30 bp DNA deletion in the EBV-LMP1 gene, initiating at codon 342, was detected in 94.4% of EBVpositive cases. By RT-PCR and western blotting, EBV-LMP1 mRNA and protein expressions were absent in all cases, re-gardless of DNA deletion. No significant differences in TGF-bata1, TGF-betaRII, p21, NF-kappaB, E2F1, or thymidylate synthase expression were identified. However, the decreased expression of p16 was found in 84.2% of EBV-positive carcinomas, relative to only 57.5% of EBV-negative tumors (p=0.024).
CONCLUSION
EBV-LMP1 DNA deletion, mRNA and protein losses are highly prevalent in EBV-positive gastric adenocarcinoma among Korean patients, along with decreased p16 expression.
- Extranodal NK/T Cell Lymphoma Accompanied by Heavy Eosinophilic Infiltration and Peripheral Blood Eosinophilia, Involving Skeletal Muscles.
- Jin Ho Paik, Yoon Kyung Jeon, Heounjeong Go, Chul Woo Kim
- Korean J Pathol. 2011;45:S70-S74.
- DOI: https://doi.org/10.4132/KoreanJPathol.2011.45.S1.S70
- 4,736 View
- 42 Download
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF - The patient was a 52-year-old female with swelling in both lower legs and peripheral blood eosinophilia. Biopsy specimen revealed the heavy infiltration of eosinophils with sparse small lymphocytes showing mild atypia. The diagnosis was Kimura disease. The symptoms including eosinophilia were relieved by steroid treatment. At 17 months from initial biopsy, the patient developed swelling of the buttock. At 25 months, fever and dyspnea with multiple lung nodules developed. Wedge resection revealed multiple aggregates of CD3(+), CD56(+), Epstein-Barr virus(+) large atypical lymphocytes with necrosis. The patient was finally diagnosed with extranodal NK/T cell lymphoma (NKTL). Epstein-Barr virus in situ hybridization retrospectively performed on the previous biopsies demonstrated Epstein-Barr virus infection in small CD3(+) lymphocytes. The patient expired after 26 months despite chemotherapy. Blood eosinophilia correlated well with disease activity during the clinical course. This case shows not only unusual histologic features, which hampered the correct diagnosis, but also a unique clinical manifestation of NKTL.
-
Citations
Citations to this article as recorded by- A case of extranodal NK/T cell lymphoma suspected to be focal myositis of the masseter muscle
Junya YAMASHITA, Daisuke TAKEDA, Tatsuya SHIRAI, Kaito URYU, Nanae YATAGAI, Masaya AKASHI
Japanese Journal of Oral and Maxillofacial Surgery.2025; 71(1): 33. CrossRef - Muscular involvement of extranodal natural killer/T cell lymphoma misdiagnosed as polymyositis: A case report and review of literature
Li-Hui Liu, Qing Huang, Yun-Hai Liu, Jie Yang, Han Fu, Lin Jin
World Journal of Clinical Cases.2020; 8(5): 963. CrossRef - Extranodal natural killer/T-cell lymphoma with paraneoplastic eosinophilic myositis
Jayati Mallick, Jasmine Zain, Dennis D. Weisenburger
Human Pathology: Case Reports.2020; 21: 200391. CrossRef - Extranodal NK/T-cell Lymphoma Mimicking Granulomatous Myositis
Norihiko Kawaguchi, Rumiko Izumi, Masahiro Kobayashi, Maki Tateyama, Naoki Suzuki, Fumiyoshi Fujishima, Juichi Fujimori, Masashi Aoki, Ichiro Nakashima
Internal Medicine.2019; 58(2): 277. CrossRef - Uveitis and Myositis as Immune Complications in Chemorefractory NK/T-Cell Nasal-Type Lymphoma Successfully Treated with Allogeneic Stem-Cell Transplant
Maria José Gómez-Crespo, Aránzazu García-Raso, Jose Luis López-Lorenzo, Teresa Villaescusa, María Rodríguez-Pinilla, José Fortes, Cristina Serrano, Salma Machan, Pilar Llamas, Raúl Córdoba
Case Reports in Hematology.2016; 2016: 1. CrossRef - Prognostic implications of CD30 expression in extranodal natural killer/T-cell lymphoma according to treatment modalities
Wook Youn Kim, Soo Jeong Nam, Sehui Kim, Tae Min Kim, Dae Seog Heo, Chul-Woo Kim, Yoon Kyung Jeon
Leukemia & Lymphoma.2015; 56(6): 1778. CrossRef - Unusual case of metachronous EBV‐associated B‐cell and NK/T‐cell lymphoma mimicking polymyositis‐diagnostic challenges and pitfalls
Esther H.L. Chan, Suat‐Jin Lu, Fredrik Petersson, Kong‐Bing Tan, Wee‐Joo Chng, Siok‐Bian Ng
American Journal of Hematology.2014; 89(1): 110. CrossRef - CD30+ extranodal natural killer/T-cell lymphoma mimicking phlegmonous myositis: A case report
YAN-JIA YANG, YA-XIN LI, YAN-BIN LIU, MEI YANG, KAI LIU
Oncology Letters.2014; 7(5): 1419. CrossRef
- A case of extranodal NK/T cell lymphoma suspected to be focal myositis of the masseter muscle
- Diffuse Large B-Cell Lymphoma Associated with Chronic Inflammation Manifested as a Soft Tissue Mass: Incidental Discovery on Histological Examination.
- Sang Yun Ha, Yoon La Choi, Sung Joo Kim, Young Hye Ko
- Korean J Pathol. 2011;45(4):417-422.
- DOI: https://doi.org/10.4132/KoreanJPathol.2011.45.4.417
- 5,334 View
- 39 Download
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF - We report an extraordinary case of diffuse large B-cell lymphoma arising in a cystic necrotic mass in a 35-year-old man who presented with a soft tissue mass at the site of previous surgery. A benign mass was surgically removed 17 years ago, after which a cystic lesion gradually developed at the same site. The resected mass appeared as a thick-walled cyst filled with brown necrotic and hemorrhagic material. On microscopic examination, the cyst wall was primarily necrotic tissue with some aggregates of large atypical lymphoid cells. These atypical cells were diffusely positive for CD20 and showed a high proliferation index, Epstein-Barr virus positivity, and clonal rearrangement of the immunoglobulin gene. His present condition was diagnosed as Epstein-Barr virus-associated diffuse large B-cell lymphoma arising from chronic inflammation. It is important to be aware of the clinical manifestations and histological features of this rare disease in light of diagnosis and treatment.
-
Citations
Citations to this article as recorded by- EBV-negative Fibrin-Associated Large B-Cell Lymphoma Arising in Thyroid Hyperplastic Nodule: Report of a Case and Literature Review
Tin Wai Ho, Wah Cheuk, John K.C. Chan
International Journal of Surgical Pathology.2023; 31(7): 1420. CrossRef - Diffuse Large B Cell Lymphoma in a Prosthetic Aortic Graft
David Bell, David Marshman
Heart, Lung and Circulation.2017; 26(2): e4. CrossRef - Fibrin-associated EBV-positive Large B-Cell Lymphoma
Daniel F. Boyer, Penelope A. McKelvie, Laurence de Leval, Kerstin L. Edlefsen, Young-Hyeh Ko, Zachary A. Aberman, Alexandra E. Kovach, Aneal Masih, Ha T. Nishino, Lawrence M. Weiss, Alan K. Meeker, Valentina Nardi, Maryknoll Palisoc, Lina Shao, Stefania P
American Journal of Surgical Pathology.2017; 41(3): 299. CrossRef - Malignant Lymphoma Mimicking an Infection After Shoulder Surgery
Jabari Ian Justin Martin, Jasmine Bauknight, Vincent Desiderio, Bahman Sadr
Journal of the American Academy of Orthopaedic Surgeons.2017; 25(4): 314. CrossRef - Current Concepts in Primary Effusion Lymphoma and Other Effusion-Based Lymphomas
Yoonjung Kim, Chan Jeong Park, Jin Roh, Jooryung Huh
Korean Journal of Pathology.2014; 48(2): 81. CrossRef
- EBV-negative Fibrin-Associated Large B-Cell Lymphoma Arising in Thyroid Hyperplastic Nodule: Report of a Case and Literature Review
- Epstein-Barr virus-associated Inflammatory Pseudotumor-like Follicular Dendritic Cell Tumor in the Spleen of a Patient with Diffuse Large B Cell Lymphoma: A Case Report and Review of the Literature.
- Sun Och Yoon, Hyoungsuk Ko, Baek hui Kim, Ghee Young Kwon, Yoon Kyung Jeon, Chul Woo Kim
- Korean J Pathol. 2007;41(3):198-202.
- 2,450 View
- 35 Download
-
 Abstract
Abstract
 PDF
PDF - We report a case of an Epstein-Barr virus (EBV)-associated inflammatory pseudotumor-like follicular dendritic cell tumor (IPT-like FDC tumor). The tumor occurred in the spleen of a 64-year-old woman with a history of a diffuse large B-cell lymphoma (DLBCL) of neck nodes that presented four years ago. The splenectomy specimen revealed a 5 cm-sized, tan-colored and well-circumscribed mass. Histologically, spindle or ovoid cells with large vesicular nuclei were admixed with abundant inflammatory cells. Immunohistochemically, spindle cells were positive for FDC marker CD35, but negative for CD20, CD30 and ALK. EBV was detected almost exclusively in spindle cells by EBER in situ hybridization. IPT-like FDC tumors are rare, and are recognized as a distinctive clinicopathologic variant of FDC tumors. Among only 18 similar cases reported in the English language literature, the present case is the first case of a patient with a history of DLBCL.
- Expression of Cancer-Related Genes in Epstein Barr Virus-Infected Burkitt's Lymphoma Cell Line Treated with Mitomycin C.
- Woo Bom Yeom, Seol Hee Park, Min Kyung Kim, Chul Hwan Kim, In Sun Kim, Dale Lee
- Korean J Pathol. 2001;35(4):271-277.
- 2,138 View
- 19 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Infection of Epstein Barr virus (EBV) into B cells drives the infected cells into the cell cycle and frequently results in lymphoblastoid cells. Mitomycin C inhibits DNA synthesis of epithelial cells as well as lymphoid cells by cross-linking with DNA. Many of the cancer cells have various pathways for escaping the responsiveness to the negative growth-regulatory effects of mitomycin C and gaining the immortalized property. The auther performed a cell culture of an EBV infected Jijoye lymphoma cell line, and compared the cell cycle and cancer related genes between the mitomycin treated- and non-treated group.
METHODS
DNA and RNA were extracted from the Jijoye cells; and EBV nuclear antigen (EBNA)-1, 2 and latent membrane protein (LMP) of EBV and p53 and p21 mRNA analyse was performed.
RESULTS
Mitomycin C blocked G2/M phase, however, mitomycin did not affect the expression of EBNA-1, 2 and LMP. Mitomycin C also increased the p21 mRNA expression without p53 mRNA increase.
CONCLUSIONS
Mitomycin C induces B cell apoptosis by blocking the G2/M phase and by increasing p21 mRNA independent to p53, which reveals the presence of an alternative pathway of p21 induction by mitomycin C in EBV positive lymphoma cells
- Detection of Epstein-Barr virus in the inflammatory and neoplastic uterine cervical lesions.
- Hye Jin Jeong, Eung Seok Lee, Zhen Hua Lin, Seol Hee Park, In Sun Kim, Jae Sung Kang
- J Pathol Transl Med. 2001;12(2):73-80.
- 2,064 View
- 18 Download
-
 Abstract
Abstract
 PDF
PDF - The prevalence of Epstein-Barr virus(EBV) in the uterine cervix was investigated to define the possible etiologic role in cervical carcinogenesis. The viral genotyping and LMP-1 30bp deletion were also studied. The materials included 169 uterine cervical swabs(152 within normal limits, 12 atypical squamous cells of uncertain significance, 3 low grade intraepithelial lesions, and 2 high grade squamous intraepithelial lesion) and 104 uterine cervical tissues obtained from hysterectomy specimens(32 carcinoma in situ, 9 microinvasive squamous cell carcinomas, 37 invasive squamous cell carcinomas, 7 adenocarcinomas, 7 adenosquamous carcinomas, and 12 cervicitis). EBV detected by PCR for EBNA-1 was positive in 52(56.5%) of 92 invasive and noninvasive cervical carcinomas, and 80(48.8%) of 164 inflammatory or normal cervices. The viruses detected in carcinomas were all type A, and LMP-1 30bp deletion form was more frequent in premalignant and malignant cervical lesions than in nonneoplastic cervices. From the above results, it may be concluded that EBV is one of common viruses detected in uterine cervix of Korean women, and type A virus and LMP-1 30bp deletion form may have a role in cervical carcinogenesis.
- Predictive Factors of Epstein-Barr Virus Association in Gastric Adenocarcinoma.
- Young Su Kim, Sang Chul Nam, Man Hoon Han, Ji Yun Jeong, Sun Kyun Park, In Soo Suh, Han Ik Bae
- Korean J Pathol. 2008;42(4):193-197.
- 2,279 View
- 18 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
It is important to detect Epstein-Barr virus (EBV) in the setting of gastric cancer so that early viral targeted therapy and prevention can be undertaken. The aim of this study was to investigate the predictive clinicopathologic factors for EBV-related gastric cancer.
METHODS
The archival tumor tissues from 335 patients with gastric cancer were examined using tissue microarray. The detection of EBV was performed using EBV mRNA in situ hybridization (EBV-ISH), and the results were compared against clinicopathologic factors.
RESULTS
EBV-related gastric cancers were identified in 21 of 335 investigated cases (6.27%). The anatomical predisposition of EBV-related cancers to manifest in the upper stomach was statistically significant (p<0.001). EBV-related cancers were almost always (20/21) accompanied by lymphoid stroma. No differences in age, sex, histologic differentiation, or T or N stage were noted between EBV-positive and EBV-negative gastric carcinomas.
CONCLUSIONS
The association of EBV with gastric adenocarcinomas could be predicted when tumors with lymphoid stroma occurred in the upper stomach.
- Characteristics of the Immortalized Human B-cells by Epstein-Barr Virus.
- Ho Jong Jeon, Bong Nam Choi, Yoon Kyeong Oh
- Korean J Pathol. 1997;31(9):832-846.
- 2,365 View
- 33 Download
-
 Abstract
Abstract
 PDF
PDF - Human lymphoblastoid B-cell lines immortalized by Epstein-Barr virus (EBV) were established from peripheral blood of patients with acute myeloblastic and chronic lymphocytic leukemia and chronic fatigue syndrome. The sera of patients with acute myeloblastic and chronic lymphocytic leukemia did not show antibodies to Epstein-Barr viral capsid antigen (VCA), but serum of a patient with chronic fatigue syndrome disclosed antibodies to VCA (IgG, IgM), and EBNA was demonstrated in peripheral blood mononuclear cells by polymerase chain reaction. The established cell lines were mature B-cell phenotypes with polyclonal proliferation in early passage and no evidence for commitment to other lineages. The immortalized cells by EBV were designated as CSUP-1 and CSUP-2 (from acute myeloblastic leukemia, FAB classification M2 and M1), CSUP-3 (from chronic lymphocytic leukemia) and CSUP-4 (from a patient with chronic fatigue syndrome). The CSUP-1, 2, 3, and 4 grew in suspension forming clumps with a doubling time of 38 to 49 hours. Colony formation was not recognized in plate. By light and electron microscopic examination, the immortalized cells showed features of lymphoblastoid to plasmacytoid lymphocytes, and multinucleated giant cells. The lymphoblastoid cells showed scanty cytoplasm with poorly developed organelles. Immunophenotypic analyses of CUSP-1, 2, 3, and 4 with monoclonal antibodies by flow cytometry showed B-cell phenotype with polyclonal proliferation in early passage. Epstein-Barr virus nuclear antigen was confirmed in the extracted DNAs from immortalized cells by polymerase chain reaction. DNA analysis showed a normodiploid stemline with a DNA index of 1.12. The established cells were strongly reactive for CD10, CD30 (Ki-1) in early passage, and bcl-2 and c-myc onco-protein in early and late passage. Karyotypic analysis of CSUP-1, 2, 3 and 4 showed 46, XY or 46, XX. No tumorigenesis in heterotransplanted SCID mouse was recognized. This immortalized cells by EBV should be a valuable cell lines to study the pathogenesis of EBV-related malignant lymphoma.
- Detection and Subtyping of Epstein-Barr Virus in Gastrointestinal Adenocarcinomas and Malignant Lymphomas.
- Young Sik Kim, Seol Hee Park, In sun Kim
- Korean J Pathol. 1997;31(9):847-861.
- 2,092 View
- 18 Download
-
 Abstract
Abstract
 PDF
PDF - Epstein-Barr virus (EBV) has been linked to a spectrum of neoplastic conditions, including Burkitt's lymphoma, nasopharyngeal carcinoma, Hodgkin's disease, lymphoepithelioma-like carcinomas and malignant lymphomas in immunocompromised state. To determine the prevalence and the subtype of EBV in gatrointestinal malignancies, fifty cases of adenocarcinomas and seventeen cases of malignant lymphomas were analyzed by EBERs in situ hybridization and polymerase chain reaction using primers for EBNA-1, EBNA-2A and EBNA-2B, on the paraffin sections. In addition, immunohistochemical stain for p53 protein was performed to investigate the potential role of EBV infection on tumor suppressor gene, p53, during tumorigenesis. EBER was detected in 6 of 26 gastric adenocarcinomas, 2 of 24 colon adenocarcinomas, and 8 of 17 malignant lymphomas. EBER was more prevalent in malignant lymphoma arising in the intestine (6/6) than in the stomach (2/11), and was detected in both B and T cell phenotypes. EBNA-1 was positive in 11 of 16 EBER positive cases and the subtyping was possible in 8; both type 1 and 2 were detected in gastric cancers, whereas only type 2 was found in intestinal neoplasms. In adenocarcinomas the high rate of p53 protein overexpression was found in both EBER positive (8/8) and negative cases (32/42), whereas the positive rate was higher in EBER positive cases (7/8) than in EBER negative cases (4/9) of malignant lymphomas. From the results, it can be concluded that EBV infection and the p53 tumor suppressor gene are independently associated in a significant portion of the gastrointestinal malignancies, but the mechanism of action remains to be elucidated.
- The Studies of bcl-2 Oncoprotein and Epstein-Barr Virus Expression in Malignant Lymphomas: Immunohistochemical and in situ hybridization analysis on 66 cases.
- Hye Jae Cho, Yeon Mee Kim, Hyun Ju Yoo, Jong Eun Joo
- Korean J Pathol. 1996;30(2):121-131.
- 2,001 View
- 12 Download
-
 Abstract
Abstract
 PDF
PDF - Bcl-2 oncoprotein is being localized to mitochondria and interfering with programmed cell death (apoptosis) independent of promoting cell division in the lymphoid and nonlymphoid cells. The bcl-2 oncoprotein expression has been reported in follicular lymphomas as well as in diffuse non-Hodgkin's lymphoma, leukemia and a variable propotion of Hodgkin's lymphoma cases. Recent evidence suggests that some lymphomas protected from apoptosis is conferred through expression of Epstein-Barr virus(EBV) latent membrane protein which turn to cause upregulation of bcl-2. To define the role of the bcl-2 oncoprotein and EBV in lymphoid malignancy, we tried immunohistochemical studies with anti-bcl-2 antibody and In situ hybridization (ISH) with EBV-encoded small nuclear RNAs(EBER) in the paraffin embedded sections of 46 non-Hodgkin's lymphoma (NHL) cases and 20 Hodgkin's lymphoma (HL) cases. Bcl-2 oncoprotein expression was found in 37 of 46 cases (80%) of NHL with relatively strong cytoplasmic staining, and in 14 of 20 cases (70%) of HL with weak cytoplasmic staining in limited small numbers of RS, Hodgkin and lacunar cells. The widespread presence of bcl-2 oncogene in many different types of both NHL and HL supports that the extended cell survival through overexpression of bcl-2 gene protein may be a growth advantage of neoplastic lymphoid cells. In the ISH analysis for EBV, the presence of EBV was detected in 17 of 20 cases (85%) of HL, compared to 6 of 44 cases(13.6%) of NHL. It appears to be no direct correlation between overexpression of bcl-2 oncoprotein by neoplastic lymphoid cells and the presence of EBV in NHL but it seems to be a definite association between EBV and HL.
- Histopathologic Analysis of Malignant Lymphoma Involving the Skin and Its Relationship with the Epstein-Barr Virus.
- Yun Hee Jin, Seong Ho Kim, Chan Kum Park
- Korean J Pathol. 2000;34(1):20-28.
- 1,990 View
- 13 Download
-
 Abstract
Abstract
 PDF
PDF - The author classified 38 cases of malignant lymphoma involving the skin primarily or secondarily by the new WHO classification with minor modifications and carried out RNA in situ hybridization and/or polymerase chain reaction (PCR) to investigate the role of Epstein-Barr virus (EBV). A case was follicular lymphoma of B cell origin and 37 cases were malignant lymphomas of T cell origin, including 15 cases of Mycosis fungoides/Sezary syndrome, five cases of subcutaneous panniculitis-like T cell lymphomas, a case of anaplastic large cell lymphoma, and four cases of primary cutaneous CD30 T cell lymphoproliferative disorders. There were eight cases of unspecified peripheral T cell lymphomas, in which four cases were composed of medium-sized cells, three cases of large cells, and a case of lymphoepithelioid cells. Four cases of nasal and nasal type NK/T cell lymphomas and three cases of unspecified peripheral T cell lymphomas showed EBV genome. The nasal and nasal type NK/T cell lymphomas, especially those involving the nasal cavity, showed close association with the EBV infection.
- Epstein-Barr Virus Associated Lymphoepithelial Carcinoma of the Parotid Gland: A case report.
- Kwang Il Kim, Young Sik Kim, In Sun Kim
- Korean J Pathol. 1998;32(2):150-152.
- 2,283 View
- 21 Download
-
 Abstract
Abstract
 PDF
PDF - Lymphoepithelial carcinoma is a rare subtype of undifferentiated carcinoma in the salivary gland. The incidence of lymphoepithelial carcinoma is about 0.4% among the patients with major salivary gland tumors. It has a racial preference; about 75% of the patients are of Mongolian ancestry. We report a case of lymphoepithelial carcinoma arising in the left parotid gland of a 52-year-old man. Grossly, the tumor was relatively well demarcated, gray-white, and solid. Microscopically, the irregular shaped syncytial tumor cell islands were evident within lymphoplasma cell-rich and desmoplastic stroma. The carcinoma cells had large vesicular nuclei and prominent nucleoli. The tumor invaded the surrounding salivary gland tissue. Epstein-Barr virus (EBV) was demonstrated by in situ hybridization for EBV-encoded RNA-1 (EBER-1) and polymerase chain reaction for EBV nuclear antigen-1 (EBNA-1).
- Application of Epstein-Barr Virus Cell Lines (CCL85 EB-3) in Performing the EBER mRNA In Situ Hybridization as a Positive Control.
- Sung Sook Kim, Woon Sup Han, Joo Young Suh, Joo Ryung Huh
- J Pathol Transl Med. 1996;7(1):38-43.
- 2,020 View
- 20 Download
-
 Abstract
Abstract
 PDF
PDF - Epstein-Barr virus(EBV) is associated with a wide spectrum of benign and malignant disorders including leukoplakia, Hodgkin's lymphoma, central nervous system lymphoma, peripheral T cell lymphoma and nasopharyngeal undifferentiated carcinoma. There are several distinctive aspects of biology of the virus that are important in investigation of virus in clinical specimens. The abundant expression of the EBER mRNA transcripts makes possible the sensitive detection of latent expression in EBV-associated tumors. Although there has been a dramatic increased interest in the direct characterization of EBV in clinical specimens, there have been few studies about the effective and reliable positive controls in performing in situ hybridization technique for EBV, especially on paraffin-embedded tissue. We applied Burkitts lymphoma cell line as positive control in EBV in hydridization using Oncor Kit. The cell block of Burkitt lymphoma cell line(CCL85 EB-3) showed strong and specific positivity for EBER in situ in nuclei of EBV infected cells.
- EBV in Situ Hybridization Study for Cutaneous T-Cell Lymphomas.
- Chan Kum Park, Chang Woo Lee, Jung Dal Lee
- Korean J Pathol. 1996;30(8):699-705.
- 2,045 View
- 19 Download
-
 Abstract
Abstract
 PDF
PDF - We studied 24 cases of cutaneous T-cell lymphomas and six cases of benign lymphoproliferative diseases of the skin (2 Jessner's lymphocytic infiltration, 2 pseudolymphoma, 2 lymphomatoid papulosis) for the presence of Epstein-Barr Virus(EBV) RNA, using the in situ hybridization(ISH) method. Among the 24 cases of cutaneous T-cell lymphomas (CTCL), 18 cases including 12 cases of mycosis fungoides(MF) were primary CTCL, and the other 6 cases were secondary CTCL. The ISH study demonstrated a positive reaction for EBER probe in 6 out of the 24 cases(25%) of CTCL, and a negative reaction for BHLF nuclear RNA probe in all the cases studied. Double-labelling immunohistochemistry/ISH studies revealed that the EBV positive cells were CD45RO positive and CD20 negative. EBV genome was not demonstrated in any benign lymphoproliferative diseases of the skin. Among the EBER positive cases, none of the 12 cases of MF demonstrated EBER signals, and 6 out of the 12(50%) cases of CTCL were positive for EBER probe. In conclusion, latent infection of EBV may play a role in the development of non-mycosis fungoides T-cell lymphomas involving the skin.
- Establishment and Characterization of an Epstein-Barr Virus-negative B-cell Line from a Patient with Dissemination of Peripheral Blood and Bone Marrow by Malignant Lymphoid Cell.
- Ho Jong Jeon, Mi Ja Lee, Yu Kyung Jeong, Yoo Hwan Park, Choon Hae Chung, Yoon Kyung Oh, Chul Heel Choi, Sang Woo Cheong
- Korean J Pathol. 1996;30(9):792-809.
- 2,029 View
- 12 Download
-
 Abstract
Abstract
 PDF
PDF - A human malignant lymphoid cell line(JeKo-1) was established from a Korean patient with retroperitoneal tumor presenting peripheral blood and bone marrow involvement by malignant lymphoid cells. This cell line was established from peripheral blood, and the cell line had the identical immunophenotypic features as malignant cells from the peripheral blood. The established cell line had features of a mature B-cell phenotype with no evidence for commitment to other lineages. The JeKo-1 grows in suspension with a doubling time of 33 hours. By light and electron microscopic examination, the established cells had a follicular center showing, a small, cleaved, lymphoid appearance, and had a large amount of cytoplasm containing few vacuoles and an irregular cytoplasmic membrane. Immunophenotypic analyses with monoclonal antibodies using flow cytometry showed a monoclonal IgM kappa and CD5- B-cell phenotype. The cells were non-reactive for T-cells and myeloid/monocyte antigens, and no evidence of Epstein-Barr virus nuclear antigen by polymerase chain reaction. DNA analysis showed a hypodiploid stemline with a DNA index of 0.83. The established cells were strongly reactive for bcl-2 and c-myc onco-protein, but lacked expression of multidrug resistance gene protein, p-glycoprotein by Western blot analysis. Karyotypic analysis of JeKo-1 showed 40-41 chromosomes. This cell line should be a valuable tool to study the dissemination of malignant lymphoma into the peripheral blood and bone marrow.
- Deletion within LMP-1 Oncogene in Hodgkin's Disease in Korea.
- Ghee Young Kwon, Woo Sung Ahn, Bo Young Lee, Seung Sook Lee, Jooryung Huh, Chul Woo Kim
- Korean J Pathol. 1998;32(9):638-646.
- 1,909 View
- 10 Download
-
 Abstract
Abstract
- LMP (latent membrane protein)-1 protein is one of the Epstein-Barr viral proteins and it is the most crucial one for the transforming activity. It is known to show considerable variation in its nucleic acid sequence and some biologic difference is reported to be associated with the variation. Twenty four cases of the EBV-associated Hodgkin's disease cases were searched for the 30-bp deletion within the C terminal intracytoplasmic domain of LMP-1 oncogene, one of the well-known genetic variation, by PCR and Southern blot using selected sets of primers and probes. The strain of the virus was also determined with PCR. Each case was positive both on LMP-1 immunostaining and in situ hybridization for EBER (Epstein-Barr encoded RNA). Deletion within LMP-1 oncogene was identified in 22 cases (92%), of which 5 cases showed wild form as well as a deleted form of LMP-1 at the same specimens. In seven cases showing the non-deleted form, pure or mixed with a deleted form, the distribution of sex and age was similar to that of the deleted form-only-group, but there was a slight tendency for a higher stage at presentation (4 of the 7 cases presented with stage IV). Those seven cases comprised of 4 cases of nodular sclerosis (NS), 2 cases of mixed cellularity (MC) and a case of lymphocyte depletion subtype while there were 9 and 12 cases of NS and MC among all the examined cases, respectively. Two cases with both a deleted form and the non-deleted form of LMP-1 showed type I and II strain of the virus while all the others contained only type of the. In conclusion, the rate of deletion in LMP-1 oncogene in our series was higher than that reported in western countries and there was a slight tendency for higher stages in cases detecting mixed deleted and non-deleted forms of LMP-1 than in cases a of deleted from of LMP-1.
- Lymphoepithelioma-like Carcinomas of the Stomach Report of 4 cases associated with Epstein-Barr virus.
- Eun Sook Nam, Duck Hwan Kim, Hye Kyung Ahn, Hyung Sik Shin, Young Sik Kim, Han Kyum Kim, Insun Kim
- Korean J Pathol. 1998;32(9):680-686.
- 2,327 View
- 10 Download
-
 Abstract
Abstract
- Lymphoepithelioma-like carcinoma (LELC) that histologically resembles nasopharyngeal lymphoepithelioma has been reported in various sites including the stomach, salivary gland, lung, skin, thymus, tonsil and uterine cervix. LELC of the stomach was rarely reported after the first report by Burke et al. in 1990. More than 60% of them were associated with Epstein-Barr virus (EBV). Most commonly affecting elderly Asians with slight male predominance (M/F ; 1.2/1), it usually is located in the proximal portion of the stomach and distinguished from lymphoid-rich adenocarcinoma by the absence of definitive glandular differentiation in the LELC. We recently experienced 4 cases of LELC of the stomach associated with EBV. Patients consisted of two Korean females and two Korean males with one in 3rd decade, one in 5th decade and two in 6th decade. The tumors of all cases were located in the proximal portion of the stomach. Gross types were 1 Borrman type I, 2 Borrman type II and 1 early gastric carcinoma type IIc. The size of the tumors varied from 0.8 cm to 7 cm. Microscopic findings were similar in all 4 tumors.; The tumors were composed of syncytial nests of undifferentiated cells having vesicular nuclei with prominent nucleoli, admixed with abundant lymphoplasma cell infiltration in the stroma. Immunohistochemical staining revealed that the tumor cells were reactive for cytokeratin and the stromal lymphocytes were mostly T cells. There were dark hybridization signals in the nuclei of most of the tumor cells but no signals in the stromal lymphocytes in three cases on in situ PCR hybridization and on all cases PCR amplification for EBNA-1. It is concluded that LELCs of the stomach have distinctive histologic characteristics and the usual association with EBV. Further accumulation of these cases will define the prognosis.

 E-submission
E-submission
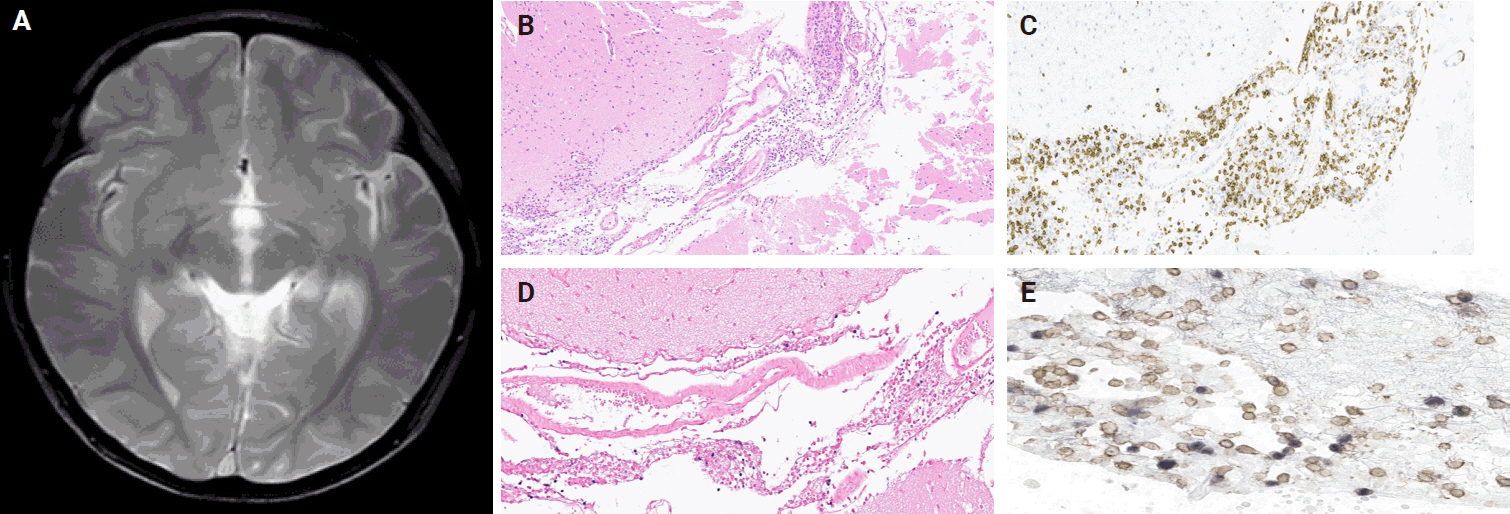






 First
First Prev
Prev



