Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 56(3); 2022 > Article
-
Newsletter
What’s new in breast pathology 2022: WHO 5th edition and biomarker updates -
Kristen Muller1
 , Julie M. Jorns2
, Julie M. Jorns2 , Gary Tozbikian3
, Gary Tozbikian3
-
Journal of Pathology and Translational Medicine 2022;56(3):170-171.
DOI: https://doi.org/10.4132/jptm.2022.04.25
Published online: May 15, 2022
1Department of Pathology and Laboratory Medicine, Dartmouth-Hitchcock Medical Center, Lebanon, NH, USA
2Department of Pathology, Medical College of Wisconsin, Milwaukee, WI, USA
3Department of Pathology, The Ohio State University Wexner Medical Center, Columbus, OH, USA
- Corresponding Author: Kristen Muller, DO Department of Pathology and Laboratory Medicine, Dartmouth-Hitchcock Medical Center, Lebanon, NH, USA E-mail: kristen.e.muller@hitchcock.org
- This article has been published jointly, with consent, in both Journal of Pathology and Translational Medicine and PathologyOutlines.com.
© 2022 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Abstract
- RARE VARIANTS OF INVASIVE BREAST CARCINOMA OF NO SPECIAL TYPE (IBC-NST)
- INVASIVE BREAST CARCINOMA WITH MEDULLARY PATTERN
- NEUROENDOCRINE TUMORS
- WELL-DIFFERENTIATED LIPOSARCOMA IN PHYLLODES TUMOR
- MUCINOUS CYSTADENOCARCINOMA
- TALL CELL CARCINOMA WITH REVERSE POLARITY (TCCRP)
- PROGNOSTIC AND PREDICTIVE BIOMARKERS UPDATE
- Meet the Authors
- REFERENCES
Abstract
- The 5th edition WHO Classification of Breast Tumours (2019) has introduced changes to our practices. Highlights are presented below, with a focus on modifications to morphological subtype categorization. In addition, we summarize important updates to ER and PR testing made in the 2020 ASCO/CAP guidelines, and briefly discuss PD-L1 and Ki-67 testing in breast cancer.
- • The prior WHO (4th ed. 2012) classified several tumors as separate entities (listed below). Breast tumors with these “special morphological patterns” now fall under the umbrella category of IBC-NST and are no longer considered to be the following clinically distinct subtypes: oncocytic, lipid-rich, glycogen-rich, clear cell, sebaceous, carcinomas with choriocarcinomatous and pleomorphic patterns, melanocytic, and carcinomas with osteoclast-like stromal giant cells.
RARE VARIANTS OF INVASIVE BREAST CARCINOMA OF NO SPECIAL TYPE (IBC-NST)
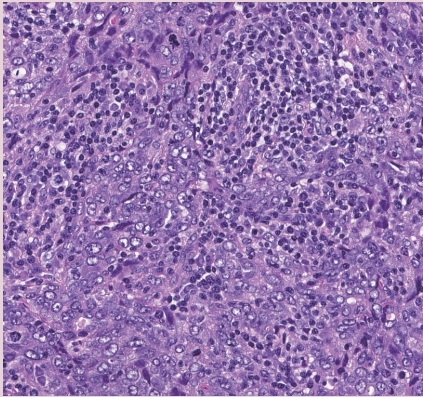
- • Medullary carcinoma, atypical medullary carcinoma, and carcinoma with medullary features were listed as special subtypes of breast carcinoma in the prior WHO. This diagnostic category has poor interobserver reproducibility; these tumors also show overlapping histologic features with carcinomas that have basal-like molecular profiles and carcinomas with BRCA1 mutations.
- • Tumor infiltrating lymphocytes (TILs) may explain the good prognosis of these cancers.
- • Carcinomas with a basal-like or medullary pattern (i.e., well-circumscribed, high-grade, syncytial architecture, necrosis, prominent TILs, Fig. 1) now represent one end of the spectrum of TIL-rich IBC-NST; “IBC-NST with medullary pattern” has been proposed to replace “medullary carcinoma.”
INVASIVE BREAST CARCINOMA WITH MEDULLARY PATTERN
- • True primary neuroendocrine (NE) neoplasms of the breast are rare. They are classified as well-differentiated NE tumors (carcinoid-like and atypical carcinoid-like) and poorly differentiated NE carcinomas (small cell neuroendocrine carcinoma and large cell neuroendocrine carcinoma).
- • Distinct NE features and expression of NE markers by IHC are needed for diagnosis, since varying degrees of NE differentiation may be seen in IBC-NST, mucinous carcinomas, solid papillary carcinomas, and others.
- • Metastasis must be ruled out before considering a primary breast NE tumor.
- • Routine staining on IBC-NST that lacks characteristic NE morphological features is not recommended, due to the lack of clinical relevance.
NEUROENDOCRINE TUMORS
- • Malignant heterologous elements are among the diagnostic criteria for malignant phyllodes tumor.
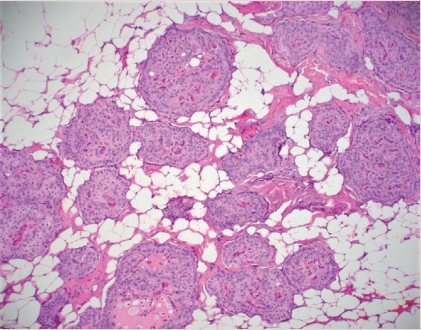
- • Adipocytic differentiation in the stromal component of phyllodes tumor, that is morphologically indistinguishable from well-differentiated liposarcoma of soft tissue, has been found to lack the characteristic MDM2 and CDK4 amplifications seen in well-differentiated liposarcoma (Fig. 2).
- • Based on recent molecular findings and evidence supporting low metastatic risk, the single criterion of well-differentiated liposarcomatous differentiation is no longer recommended for establishing the diagnosis of malignant phyllodes tumor.
WELL-DIFFERENTIATED LIPOSARCOMA IN PHYLLODES TUMOR
- • This recently described, rare, invasive breast cancer subtype is characterized by cystic spaces lined by neoplastic columnar epithelium with papillae and abundant intracellular and extracellular mucin.
- • The tumor border is rounded/encapsulated but there is lack of myoepithelium throughout.
- • Coexistent DCIS may be present and is helpful in supporting a breast origin.
MUCINOUS CYSTADENOCARCINOMA
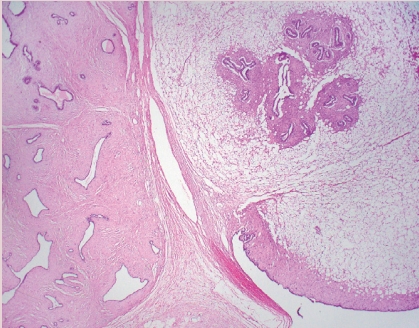
- • This recently described, rare, invasive breast cancer subtype has characteristic features that include solid nests of tumor, set in fibrous stroma, that have delicate fibrovascular cores lined by bland columnar epithelial cells (Fig. 3).
- • Cells have abundant eosinophilic cytoplasm with nuclei present at the apical poles (i.e., reverse polarity) that may have grooves and inclusions.
- • Despite low grade morphology, these tumors are positive for CK 5/6 and are triple negative for ER, PR, and HER2. IDH2 p.Arg172 hotspot mutations have been reported in 84% of tumors studied.
TALL CELL CARCINOMA WITH REVERSE POLARITY (TCCRP)
- • PD-L1 (clone 22C3)
- On July 26, 2021, the United States Food and Drug Administration (FDA) approved pembrolizumab for high-risk early-stage triple-negative breast cancer (TNBC) in combination with chemotherapy as neoadjuvant treatment; subsequently it was approved as a single agent adjuvant treatment.
- The FDA converted the accelerated approval of pembrolizumab, in combination with chemotherapy, for the treatment of locally recurrent unresectable or metastatic TNBC tumors that express PD-L1 (clone 22C3) with a combined positive score (CPS) ≥ 10.
- CPS is the number of PD-L1 staining cells (tumor cells, lymphocytes, macrophages) divided by the total number of viable tumor cells, multiplied by 100.
- • PD-L1 (clone SP142)
- On August 27, 2021, Genentech withdrew its accelerated indication for atezolizumab plus nab-paclitaxel for the treatment of PD-L1 (clone SP142) positive advanced/ metastatic TNBC.
- • Ki-67 (clone MIB-1)
- On October 13, 2021, the FDA approved abemaciclib plus endocrine therapy for hormone receptor positive, HER2 negative, node positive early breast cancer patients with a Ki-67 index ≥ 20%, who are at high risk for recurrence.
- The FDA approved companion diagnostic to abemaciclib is Ki-67 using the MIB-1 PharmDX/Dako Omnis antibody clone.
- • 2020 ASCO/CAP ER/PR Guideline Update Highlights [1]
- ER low-positive new reporting recommendation: “The cancer in this sample has a low level (1%–10%) of ER expression by IHC. There are limited data on the overall benefit of endocrine therapies for patients with low level (1%–10%) ER expression, but they currently suggest possible benefit, so patients are considered eligible for endocrine treatment. There are data that suggest invasive cancers with these results are heterogeneous in both behavior and biology and often have gene expression profiles more similar to ER-negative cancers.”
- PR testing is optional in DCIS.
- There is a new recommendation for laboratories to establish a specific protocol to ensure the validity of ER low-positive (1–10%) or negative (0 or < 1%) interpretations and results.
PROGNOSTIC AND PREDICTIVE BIOMARKERS UPDATE
- Dr. Muller has been an author for PathologyOutlines since 2020 and part of the PathologyOutlines editorial board since 2021. She is currently an Assistant Professor at Dartmouth-Hitchcock Medical Center where she practices Breast and Gynecologic Pathology.
- Dr. Jorns has been an author for PathologyOutlines since 2014 and part of the PathologyOutlines editorial board since 2020. She is currently an Associate Professor at the Medical College of Wisconsin where she primarily practices Breast Pathology.
- Dr. Tozbikian has been an author for PathologyOutlines since 2018, part of the PathologyOutlines editorial board since 2019, and Deputy Editor in Chief for Breast Pathology since 2021. He is currently an Associate Professor at The Ohio State University Wexner Medical Center where he practices Breast and Genitourinary Pathology.
Meet the Authors
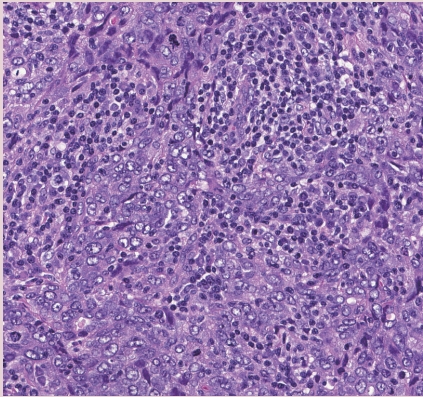
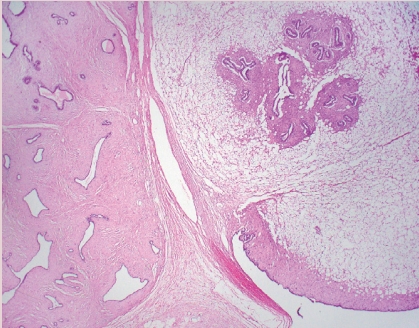
Figure & Data
References
Citations

- Comparative Evaluation of Machine Learning-Based Radiomics and Deep Learning for Breast Lesion Classification in Mammography
Alessandro Stefano, Fabiano Bini, Eleonora Giovagnoli, Mariangela Dimarco, Nicolò Lauciello, Daniela Narbonese, Giovanni Pasini, Franco Marinozzi, Giorgio Russo, Ildebrando D’Angelo
Diagnostics.2025; 15(8): 953. CrossRef - Cannabinol improves exemestane efficacy in estrogen receptor-positive breast cancer models: a comparative study with cannabidiol
Cristina Ferreira Almeida, Maria João Valente, Natércia Teixeira, Susana Rocha, Ana Paula Ribeiro, Anne Marie Vinggaard, Georgina Correia-da-Silva, Cristina Amaral
European Journal of Pharmacology.2025; 1000: 177712. CrossRef - Impact of minor cannabinoids on key pharmacological targets of estrogen receptor-positive breast cancer
Cristina Ferreira Almeida, Georgina Correia-da-Silva, Ana Paula Ribeiro, Natércia Teixeira, Cristina Amaral
Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids.2025; 1870(6): 159658. CrossRef - Reopening Pandora’s box: Is there a role for HDL in breast cancer?
Maria Isabela Bloise Alves Caldas Sawada, Monique de Fatima de Mello Santana, Milena Gomes Vancini, Marisa Passarelli
Seminars in Cancer Biology.2025; 114: 227. CrossRef - Breast cancer metastasizing to Jaw bones as the sole primary source: Systematic review
Sonia Gupta, Nausheen Aga, Aruna Vanka, Ruchira Shreevats, Muna Eisa Mohamed Hassan, Fatema Matcheswala
National Journal of Maxillofacial Surgery.2025; 16(2): 220. CrossRef - Correlation of Histopathology and Radiological Findings Among the Diverse Breast Lesions in a Tertiary Care Centre
Ranjani Mohan, Sathish Selvakumar A, Ragupathy S, Meenakshisundaram K, Shanmugapriya S, Rajeswari Kathiah, Rajeswari T, Priavadhana Rajan Prasaad, Dinesh Kumar S, Sarika K
Cureus.2024;[Epub] CrossRef - Diagnostic Challenge in Veterinary Pathology: Metastatic Mammary Tumor in a Female Tiger (Panthera Tigris)
Charisha Fraser, Mun Keong Kok, Intan Shameha Abdul Razak, Yulianna Puspitasari, Annas Salleh
Veterinary Pathology.2024; 61(4): 508. CrossRef - Expression of cell surface zinc transporter LIV1 in triple negative breast cancer is an indicator of poor prognosis and therapy failure
Roshni Saravanan, Vaishnavi Balasubramanian, Sandhya Sundaram, Bhawna Dev, Pavithra Vittalraj, Ravi Shankar Pitani, Gouthaman Shanmugasundaram, Suresh Kumar Rayala, Ganesh Venkatraman
Journal of Cellular Physiology.2024;[Epub] CrossRef - Oral Soft Tissue Metastasis from Breast Cancer as the Only Primary Source: Systematic Review
Nausheen Aga, Ruchira Shreevats, Sonia Gupta, Harman Sandhu, Muna E.M. Hassan, Harnisha V. Prajapati
Avicenna Journal of Medicine.2024; 14(01): 022. CrossRef - Influence of tumor microenvironment on the different breast cancer subtypes and applied therapies
Cristina Ferreira Almeida, Georgina Correia-da-Silva, Natércia Teixeira, Cristina Amaral
Biochemical Pharmacology.2024; 223: 116178. CrossRef - Specific feature recognition on group specific networks (SFR-GSN): a biomarker identification model for cancer stages
Bolin Chen, Yuxin Wang, Jinlei Zhang, Yourui Han, Hamza Benhammouda, Jun Bian, Ruiming Kang, Xuequn Shang
Frontiers in Genetics.2024;[Epub] CrossRef - BREAST CANCER IN THE POLTAVA REGION: CLINICAL AND MORPHOLOGICAL ASPECTS
K. R. Novykov, L. P. Lytvynenko, B. M. Fylenko, N. V. Roiko, O. K. Prylutskyi, S. A. Proskurnia
Bulletin of Problems Biology and Medicine.2024; 1(2): 9. CrossRef - Breast cancer metastasizing to salivary glands: Systematic review
Sonia Gupta, Mayur Manoharrao Shingade, Manasi Pangarkar, Annie Evangelin Nithiakumar, Pallavi Sharma, Nausheen Aga, Kinza Qureshi, Muna Eisa Mohamed Hassan, Achla Bharti Yadav
National Journal of Maxillofacial Surgery.2024; 15(2): 199. CrossRef - Granular cell tumour of the breast
Nicole Ellen James, Yue Guan, Fawaz Musa, Giulio Cuffolo
BMJ Case Reports.2024; 17(8): e258326. CrossRef - Primary extra – nodal DLBCL at rare sites: A case series
Shruti Vijayakumar, Shalini Kuruvilla, Kavitha Kanjirakkattu Mana Parameswaran, Shahin Hameed
Indian Journal of Pathology and Oncology.2024; 11(3): 289. CrossRef - Molecular Targets of Minor Cannabinoids in Breast Cancer: In Silico and In Vitro Studies
Cristina Ferreira Almeida, Andreia Palmeira, Maria João Valente, Georgina Correia-da-Silva, Anne Marie Vinggaard, Maria Emília Sousa, Natércia Teixeira, Cristina Amaral
Pharmaceuticals.2024; 17(9): 1245. CrossRef - Immune environment of high-TIL breast cancer: triple negative and hormone receptor positive HER2 negative
Su-Jin Shin, Inho Park, Heounjeong Go, Jiwon Ko, Yangkyu Lee, Jee Hung Kim, Sung Gwe Ahn, Joon Jeong, Soong June Bae, Yoon Jin Cha
npj Breast Cancer.2024;[Epub] CrossRef - Precision medicine in cancer treatment: Revolutionizing care through proteomics, genomics, and personalized therapies
Riddhi Jawdekar, Vaishnavi Mishra, Kajal Hatgoankar, Yugeshwari R. Tiwade, Nandkishor J. Bankar
Journal of Cancer Research and Therapeutics.2024; 20(6): 1687. CrossRef - Immune checkpoint inhibitor resistance in hepatocellular carcinoma
Zhijie Wang, Yichuan Wang, Peng Gao, Jin Ding
Cancer Letters.2023; 555: 216038. CrossRef - Demographic and Clinical Features of Patients with Metastatic Breast Cancer: A Retrospective Multicenter Registry Study of the Turkish Oncology Group
Izzet Dogan, Sercan Aksoy, Burcu Cakar, Gul Basaran, Ozlem Ercelep, Nil Molinas Mandel, Taner Korkmaz, Erhan Gokmen, Cem Sener, Adnan Aydiner, Pinar Saip, Yesim Eralp
Cancers.2023; 15(6): 1667. CrossRef - The role of tumor microenvironment in drug resistance: emerging technologies to unravel breast cancer heterogeneity
Vincenzo Salemme, Giorgia Centonze, Lidia Avalle, Dora Natalini, Alessio Piccolantonio, Pietro Arina, Alessandro Morellato, Ugo Ala, Daniela Taverna, Emilia Turco, Paola Defilippi
Frontiers in Oncology.2023;[Epub] CrossRef - Unraveling the Role of Adiponectin Receptors in Obesity-Related Breast Cancer
Giuseppina Daniela Naimo, Alessandro Paolì, Francesca Giordano, Martina Forestiero, Maria Luisa Panno, Sebastiano Andò, Loredana Mauro
International Journal of Molecular Sciences.2023; 24(10): 8907. CrossRef - Novel Molecular Targets for Immune Surveillance of Hepatocellular Carcinoma
Pietro Guerra, Andrea Martini, Patrizia Pontisso, Paolo Angeli
Cancers.2023; 15(14): 3629. CrossRef - Breast metastasis as the first presentation of an anorectal melanoma diagnosed on fine needle aspiration cytology: a case report
Adil Aziz Khan, Shaivy Malik, Sana Ahuja, Mukul Singh
Surgical and Experimental Pathology.2023;[Epub] CrossRef - Meme Kanserinde Ki67 İndeks Ölçümlerinin Manuel ve Dijital Yöntemler Açısından Kıyaslanması
Zuhal SİLAV
İstanbul Gelişim Üniversitesi Sağlık Bilimleri Dergisi.2023; (20): 397. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
- Related articles
-
- What’s new in hematopathology 2025: myeloid neoplasms in the WHO 5th edition and ICC
- What’s new in neuropathology 2024: CNS WHO 5th edition updates
- What’s new in adrenal gland pathology: WHO 5th edition for adrenal cortex
- What’s new in thyroid pathology 2024: updates from the new WHO classification and Bethesda system
- What’s new in genitourinary pathology 2023: WHO 5th edition updates for urinary tract, prostate, testis, and penis




 E-submission
E-submission